Efforts to Curb the Administration of These Drugs in Animal Agriculture Can Help Preserve Their Effectiveness
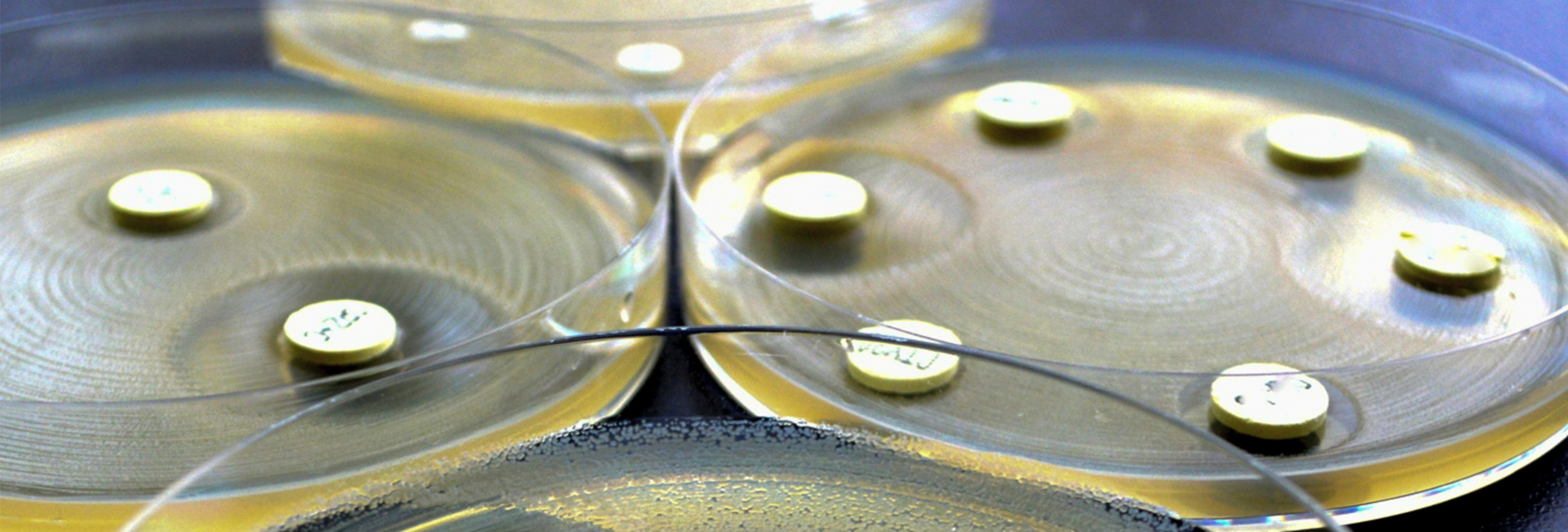
The more that antibiotics are used, the less effective they become. This includes the use of antibiotics in food animals, which helps drive the emergence of drug-resistant bacteria.
For more than a decade, Pew worked with government agencies and companies from across the food supply chain to reduce the need for antibiotics in food animals through the use of alternative products, improved management practices, and effective stewardship. Pew concluded this work in December 2021.