State Oversight of Drug Compounding
Major progress since 2015, but opportunities remain to better protect patients
Overview
More than five years have passed since contaminated injections compounded at a single pharmacy caused 76 deaths and 778 illnesses in a nationwide outbreak of fungal meningitis, a tragedy that made clear that the complex, technical practice of drug compounding was not subject to a level of oversight appropriate to its potential risks to patients. Since then, state and federal officials have been re-examining the laws and regulations governing compounding, and working to strengthen them. Compounding is the creation of medications tailored to patients whose clinical needs cannot be met by U.S. Food and Drug Administration-approved products.
Compounded medications pose a higher level of risk to patients than FDA-approved drugs because they have not been tested for safety and efficacy, have not gone through an approval process, and are typically not made under the same quality standards as approved products are. The Pew Charitable Trusts’ drug safety project has identified more than 50 reported compounding errors or potential errors from 2001 to 2017 linked to 1,227 adverse events—undesirable experiences associated with the use of a medical product—including 99 deaths. And because many such events may go unreported, this number is likely to be an underestimation.
Scrutiny of compounding policies following the meningitis outbreak in 2012 brought to light weaknesses in state and federal oversight of these potentially risky drugs, prompting reforms at both levels. In November 2013, Congress passed and President Barack Obama signed into law the bipartisan Drug Quality and Security Act (DQSA), which established clear lines of oversight accountability for two categories of businesses that can compound drugs:
- States oversee compounders of patient-specific drugs. They have primary jurisdiction over traditional compounders, who tailor medications to individual patients and include pharmacists practicing in a variety of settings, including community pharmacies and hospitals, as well as physicians who create medications for administration to their patients. These traditional compounders were placed under state jurisdiction in 1997 after Congress introduced new federal policy on compounding as part of the Food and Drug Administration Modernization Act, adding Section 503A to the Federal Food, Drug, and Cosmetic Act (FDCA), and remain so under the DQSA. Both compounding pharmacies and physicians who compound drugs in their offices can be considered traditional compounders, but this report focused on oversight of pharmacies.
- FDA oversees drugs compounded without an individual patient in mind, known as non-patient-specific compounded drugs. FDA is the primary regulator of outsourcing facilities, which can produce “office stock” (bulk supplies of non-patient-specific compounded drugs for hospitals, doctors’ offices, and other health care facilities), and are regulated under Section 503B of the FDCA.
The vast majority of compounding is patient-specific; as such, it remained under states’ jurisdiction in the federal law. In response to both the outbreak and the subsequent federal law that clarified these regulatory responsibilities, many states also began developing strategies to strengthen their own drug compounding oversight.
As state officials were seeking to determine which reforms would help them oversee the industry most effectively, Pew convened an advisory committee of state pharmacy regulators and other experts to identify best practices (see the “Best Practices” section below), which were published in its 2016 report “Best Practices for State Oversight of Drug Compounding.”
In 2016 Pew also published the report “National Assessment of State Oversight of Sterile Drug Compounding,” an evaluation of the national landscape of state policies on compounding of sterile drugs, based on data collected in 2015. The current report provides a targeted update of the prior assessment, focusing on state alignment with three key best practices:
- Application of U.S. Pharmacopeial Convention (USP) quality standards on sterile compounding.
- Harmonization with federal law on compounding without prescriptions.
- Annual inspections of facilities that perform sterile compounding.
This assessment collected data from publicly available sources, which were then verified by the boards of pharmacy in 43 states and the District of Columbia, and through interviews with representatives from four randomly selected boards.
State officials have strengthened sterile compounding oversight laws and rules since the 2015 assessment. The vast majority of states now conform to best practices in two of the three key areas:
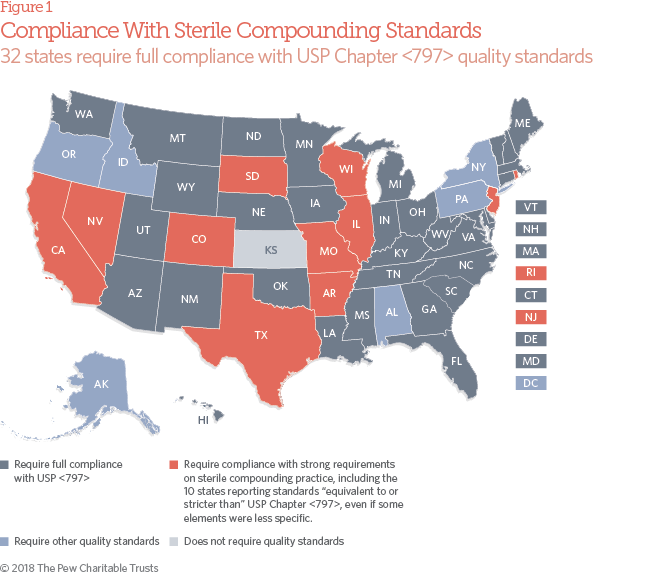
- 32 state boards of pharmacy require traditional pharmacies that compound sterile drugs for humans to be in full compliance with the widely recognized quality standards established by the USP in its General Chapter , “Pharmaceutical Compounding—Sterile Preparations.” An additional 11 states have strong requirements on sterile compounding practice, which 10 of them characterize as “equivalent to or stricter than” Chapter , even if some elements are less specific. An additional four states have pending policy changes that, if passed, would require full compliance with or other strong quality standards. In 2015, just 26 states required or equivalent quality standards for sterile compounding.
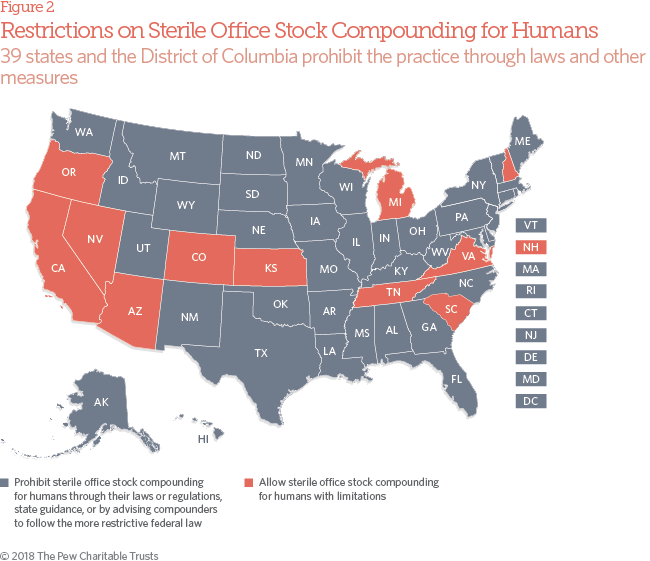
- 39 states and the District of Columbia prohibit traditional pharmacies from compounding for sterile office stock for human use—through their laws, regulations, or state guidance, or by advising compounders to follow the DQSA. However, 11 states have office stock policies (many predating the federal law) that are not aligned with federal statute. In 2015, representatives from nearly two-thirds of state boards of pharmacy that responded to the Pew assessment allowed traditional compounding pharmacies to produce drugs without prescriptions to at least some extent.
- It appears that states may be inspecting traditional pharmacies that do sterile compounding for humans less frequently now than in 2015. Then, 26 states and the District conducted routine inspections at least annually for in-state pharmacies that perform sterile compounding; today, just 22 states and the District do so. Interviews with state officials underscore the need for more financial resources and inspection capacity.
The significant progress in adopting USP Chapter quality standards and aligning with federal law on compounding without prescriptions suggests a key opportunity for jurisdictions that have not yet adopted these best practices. Improvements in rigor and frequency of inspection of facilities that perform sterile compounding will require resources, but interim measures such as harmonizing inspection forms and processes among states may allow for optimal use of existing capacity and enhance efficiencies.
While the majority of states have taken action to strengthen sterile compounding oversight policies since the outbreak, it is essential to follow through with strong implementation and enforcement of these laws and rules—including the federal DQSA. This report is intended to highlight the significant progress on public health policy that has occurred and to identify the most fruitful opportunities for action to help ensure a safe supply of compounded drugs. This remains a period of flux for drug compounding oversight: A number of states have pending policy changes, and implementation of the federal DQSA is ongoing. This continuing progress is one key finding of this study.