Patient Review and Restriction Programs in Medicaid
Expert panel recommendations to help curb misuse of prescription drugs
Overview
Opioid-use disorders pose a serious public health problem in the United States. Medicaid beneficiaries are especially at risk: Nationwide, people on Medicaid enter emergency departments for opioid-related overdoses at much higher rates than patients with other types of insurance.1
Patient review and restriction (PRR) programs are one tool that Medicaid and other payers employ to protect patients from receiving harmful amounts and combinations of opioids and other controlled substances. PRR program staff consult data describing the use of prescription drugs and medical services to identify at-risk patients—such as those receiving prescription drugs from multiple health care providers or filling multiple controlled substance prescriptions—and typically assign these individuals to obtain future prescriptions only from a designated pharmacy, or a designated prescriber and pharmacy.
A March 2016 report by The Pew Charitable Trusts, Curbing Prescription Drug Abuse With Patient Review and Restriction Programs: Learning From Medicaid Agencies,2 examined how Medicaid programs operate PRRs. The report is based on a survey Pew sent to 52 Medicaid programs in the United States, including the District of Columbia’s and Puerto Rico’s; 38 jurisdictions that operate a PRR program for their fee-for-service Medicaid program responded.3 Overall, the report found significant variability in the structure and features of these programs.
As a follow-up to this report, Pew brought together select Medicaid personnel in May 2016 to discuss these differences and develop recommendations for improving Medicaid PRRs. Panelists were selected to ensure representation of a diverse group of PRRs (see Appendix A for a list of Medicaid participants). These recommendations are based on expert opinion and the findings from the survey; further research is needed to support and develop these best practices.
The 11-member expert panel made the following recommendations for optimizing PRRs:
Recommendation 1: Medicaid PRRs should assign beneficiaries to both a pharmacy and a prescriber to obtain prescriptions for controlled substances and other drugs identified as frequently subject to misuse.
All 38 of the programs that responded to Pew’s survey restrict patients to a designated pharmacy. In addition, 25 of them (66 percent) restrict PRR enrollees to a designated prescriber.4 In pharmacy-only programs, patients may still obtain prescriptions from multiple doctors and fill them at the designated pharmacy. The prescribers may not be aware that patients are receiving opioids or other controlled substances from multiple providers, putting the patient at risk for overdose or other harm. According to expert panel participants, assigning patients to both a pharmacy and a prescriber strengthens patient-care coordination and helps to promote safe opioid use.
The panel also considered whether PRRs should include other types of providers, including a designated hospital.
Eight survey respondents designate a hospital in addition to a pharmacy and provider. Panelists agreed that this addition may further improve care coordination, but they were concerned that restricting these services could discourage people from seeking needed care.5 Assigning patients to a hospital and monitoring their use of services also requires additional staff resources that may not be available to all PRRs.
As such, panelists agreed that designating only a pharmacy and prescriber is an appropriate structure for maximizing PRR effectiveness.
Recommendation 2: Medicaid PRRs should have access to state prescription drug monitoring programs (PDMPs) and restrict the use of out-of-pocket payments for covered services.
The expert panel agreed that a weakness of PRRs—programs that depend primarily upon data derived from pharmacy insurance claims—is their inability to monitor and restrict services that are paid for out-of-pocket. Panel participants identified two ways to address this loophole: restricting the acceptance by pharmacies and providers of out-of-pocket payments for covered services and monitoring these payments through PDMPs, the state-run electronic databases that monitor all dispensed prescriptions for controlled substances. These two strategies improve the effectiveness of PRRs by reducing “doctor shopping” and patient circumvention of the program.
Panelists reported enforcing restrictions on the use of out-of-pocket payments for services such as office visits and controlled substance prescriptions through approaches including provider contracts, regulations, and policies and procedures outlined in provider manuals. When individuals attempt to circumvent the PRR by claiming they are uninsured, these policies require prescribers and pharmacists to check for Medicaid eligibility. The provider would then see that the patient was enrolled in the PRR program and comply with applicable restrictions. The expert panel recommended that Medicaid PRRs should implement out-of-pocket payment restrictions; however, the best implementation method was not determined and would probably vary by state.
PDMPs can also be used to monitor the compliance of patients, prescribers, and pharmacy staff with out-of- pocket restrictions. Members of the expert panel with access to their state’s PDMP said they have effectively used it in this capacity.6 In the absence of PDMP data, PRR program staff are restricted to information on services billed through that insurer.
The expert panel agreed that PRR program staff should have access to state PDMPs and integrate them into the PRR program. As of September 2015, however, only 32 states allow insurers, including Medicaid, to do so.7 Even in states that allow access, eight PRRs reported that they still can’t get the information.8 This may occur if Medicaid has access to the PDMP but the PRR program does not have the staff capacity to get the information from the PDMP, or if the PDMP is not yet fully operational. Panelists recommended that Medicaid staff work with state legislators and PDMP administrators to gain full access to maximize the effectiveness of the PRR.
Recommendation 3: Medicaid PRRs should offer enrollees additional services to improve overall patient care.
In addition to case management and information on substance use disorders (SUDs), other services provided by PRRs may include:
- Referrals for SUD treatment.
- Referrals to pain specialists.
- Information on the appropriate use of health care services, such as the emergency department and a primary care provider.
- Information on the importance of using only one pharmacy and/or prescriber.
- Medication therapy management.
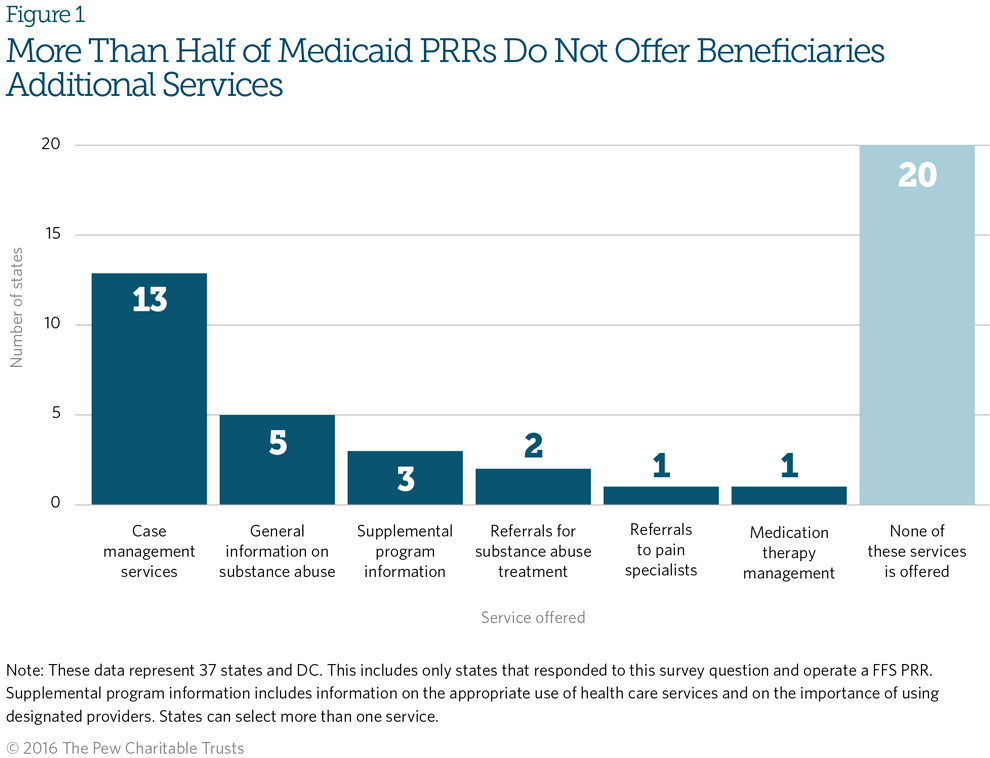
Figure 1 shows the services that the 38 Medicaid PRRs that responded to Pew’s survey offered; more than half reported that they did not offer additional services related to the patients’ use of pain management drugs.9 The panel agreed that offering case management and other services, such as referral to treatment, could help patients receive the help they may need in recovering from or recognizing a potential SUD.
The expert panel also discussed why more Medicaid PRRs are not providing these services. Possible reasons include limited program resources to refer patients and a lack of interest from patients. For example, several panelists indicated that when they have offered additional services, patients have been unresponsive or uninterested. However, with repeated outreach by program staff, patients were more likely to opt in to the additional services.
Recommendation 4: Medicaid PRRs should include a wider range of drugs with fraud or misuse potential.
The Drug Enforcement Administration classifies controlled substances in five schedules determined by whether the drug has acceptable medical uses and its potential for abuse or dependency. Schedule I includes only illicit drugs with no accepted medical use; all prescription controlled substances are in schedules II-V. Thirty-five of the 38 Medicaid PRRs that participated in Pew’s survey (92 percent) require patients to receive all controlled substances from designated pharmacies. In addition to covering all controlled substances, 17 programs (45 percent) also require patients to receive noncontrolled drugs identified by the program as frequently subject to fraud, such as those used to treat HIV,10 from designated providers.11
Expanding PRRs to include noncontrolled drugs frequently subject to misuse, such as the antiepileptic medication gabapentin, would grant the programs the flexibility to address broader patterns of drug misuse. The majority of panelists agreed that including these drugs allows the designated pharmacy to act as a safeguard for all commonly misused substances. However, one participant noted limited evidence showing that the added restriction improves health outcomes and expressed concerns about barriers to access if noncontrolled substances were included in PRRs.
Panelists indicated that the PRRs that include noncontrolled substances frequently subject to misuse should still enroll patients based only on their controlled substance prescription history. However, once patients are enrolled in the PRR, prescriptions for noncontrolled drugs subject to misuse would be treated similarly to prescriptions for controlled drugs.
Recommendation 5: Medicaid PRRs should develop best practices for enrollment criteria but allow for deviation based on state-specific factors.
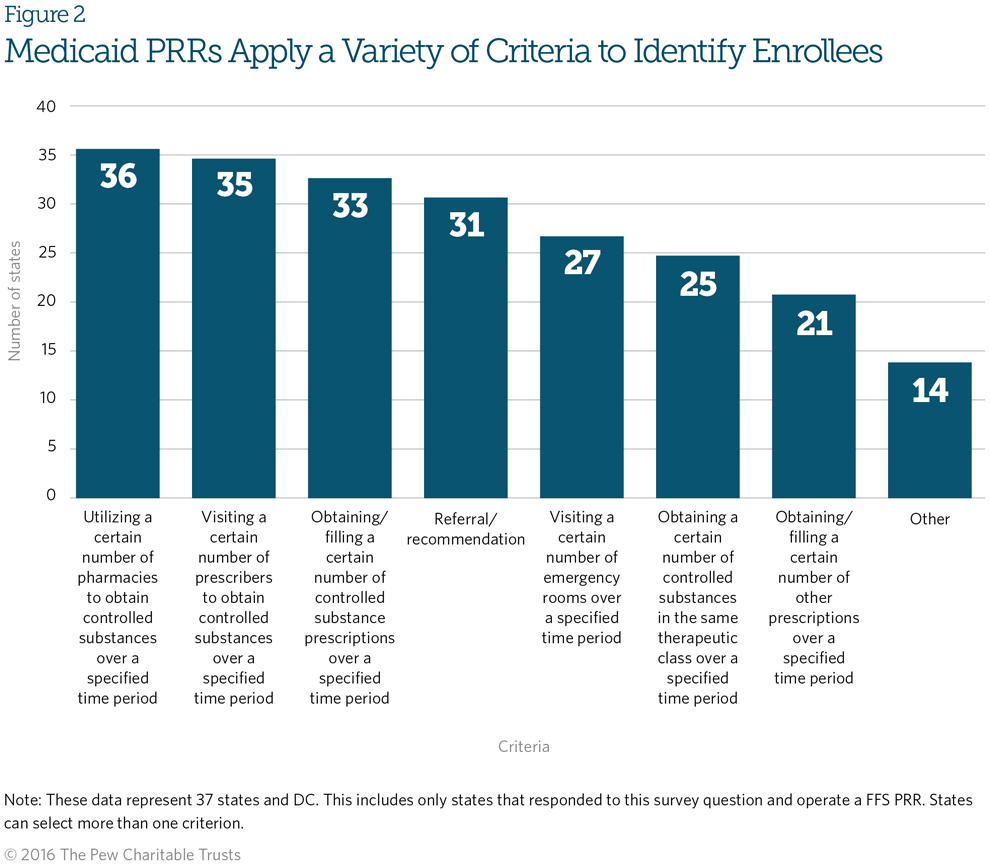
Figure 2 shows the criteria Medicaid PRRs use to identify potentially at-risk beneficiaries. While there is variation across states, the most commonly used criteria are visiting a certain number of pharmacies, seeing a certain number of prescribers, and obtaining a certain number of controlled substance prescriptions over a specified period. Twenty-nine of the PRRs that responded to Pew’s survey use all three of these criteria. Other benchmarks used include visiting a certain number of emergency rooms or obtaining a certain number of controlled substances in the same therapeutic class over a specified period.12 Overall, roughly three-quarters of PRRs use at least five criteria, and five use more than 10 criteria to identify at-risk beneficiaries.
The panel agreed on the need to determine which criteria are most effective and to create a set of standard criteria. However, panelists emphasized the need for flexibility for states to vary from the standards based on population, geographic size, the extent of prescription drug misuse and diversion, and available PRR program resources. More research is needed to build consensus on best practice criteria.
Conclusion
PRRs can improve care coordination and prevent inappropriate access to controlled substances and other medications that are susceptible to misuse. The variation in these programs across states can inform best practices, such as those identified by this expert panel. While members of the panel did not recommend complete standardization due to state variations, they agreed that Medicaid PRRs should consider these recommendations to optimize patient care and program effectiveness.
Appendix A: Panel participants
Members of the Medicaid PRR program expert panel, which met May 24, 2016, were:
Tammy Amend
Unit supervisor, provider review and lock-in Missouri Medicaid Audit and Compliance Unit
Debra Bennion
Manager, Utah Lock-in Program Utah Department of Health
Scott Best
Clinical Review and Patient Review and Coordination Unit manager
Washington State Health Care Authority
Sandra Deaver
Medicaid pharmacy case manager Wyoming Department of Health, Division of Healthcare Financing
William Gilbert
Assistant investigator in charge
New York office of the Medicaid inspector general
Kyle Huelsman
Health integration specialist
Colorado Department of Health Care Policy and Financing
Larry Humble
Director, Office of Outcomes Research and Evaluation University of Louisiana, Monroe School of Pharmacy
Kimberly Lenz
Clinical pharmacy manager
Massachusetts Division of Medical Assistance
Pamela Mailey
Director, Division of Program and Provider Compliance, Bureau of Program Integrity Pennsylvania Department of Human Services, Office of the Administration
Raymond McIntire
Director of pharmacy operations Tennessee Bureau of TennCare
Kulani Moti
Supervisor, Minnesota Restricted Recipient Program Minnesota Department of Human Services
Patti Raymond
Program manager, Washington Patient Review and Coordination Program
Washington State Health Care Authority
Melwyn Wendt
Pharmacy director
Louisiana Department of Health
Endnotes
- Centers for Disease Control and Prevention, “Patient Review and Restriction Programs: Lessons Learned From State Medicaid Programs” (2012), https://www.cdc.gov/drugoverdose/pdf/pdo_patient_review_meeting-a.pdf.
- The Pew Charitable Trusts, Curbing Prescription Drug Abuse With Patient Review and Restriction Programs: Learning From Medicaid Agencies (2016), http://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2016/03/curbing-prescription-drug-abuse-with-patient- review-and-restriction-programs.
- Twenty-eight states operate PRRs in both their Medicaid managed care and fee-for-service populations; 16 states administer PRRs only in their Medicaid fee-for-service population; and three states administer a PRR only in Medicaid managed care. Pew survey questions pertained only to fee-for-service programs.
- The Pew Charitable Trusts, Curbing Prescription Drug Abuse With Patient Review and Restriction Programs: Learning From Medicaid Agencies.
- Among other requirements, the Centers for Medicaid & Medicaid Services prohibits these programs from applying restrictions to emergency services.
- The Pew Charitable Trusts, Curbing Prescription Drug Abuse.
- National Alliance for Model State Drug Laws, 2015 Annual Review of Prescription Monitoring Programs (2015), 24, http://www.namsdl.org/ library/1810E284-A0D7-D440-C3A9A0560A1115D7.
- The Pew Charitable Trusts, Curbing Prescription Drug Abuse.
- Ibid.
- Department of Health and Human Services office of the inspector general, Part D Beneficiaries With Questionable Utilization Patterns for HIV Drugs (2014), http://oig.hhs.gov/oei/reports/oei-02-11-00170.pdf.
- The Pew Charitable Trusts, Curbing Prescription Drug Abuse.
- Ibid.


Curbing Prescription Drug Abuse With Patient Review and Restriction Programs
Public and private insurance plans are using patient review and restriction (PRR) programs to encourage the safe use of opioids and other controlled substances.
Learn More

Patient Review and Restriction Programs Curb Opioid Misuse
Medicaid programs use PRRs to address inappropriate use of prescription drugs and reduce the risk of overdose