Public Health at Risk
Failures in Oversight of Genetic Testing Laboratories
Quick Summary
The Human Genome Project unleashed a torrent of information about the human genome and the role of genetic variation in human health. As a result, genetic testing is now among the fastest growing areas of laboratory medicine. Today, genetic tests for about 1000 diseases are clinically available, with hundreds more available in a research setting.
Executive Summary
The Human Genome Project unleashed a torrent of information about the human genome and the role of genetic variation in human health. As a result, genetic testing is now among the fastest growing areas of laboratory medicine. Today, genetic tests for about 1000 diseases are clinically available, with hundreds more available in a research setting.
Making sure that laboratories can accurately and reliably perform genetic tests is a fundamental requirement for the success of genetic medicine, and the government has a key role to play in overseeing laboratory quality. Congress has provided federal agencies a broad mandate to ensure the accuracy and reliability of genetic testing, but inattention and delay have meant that this mandate has gone unheeded.
This report describes the role of the Clinical Laboratories Improvement Amendments of 1988 (CLIA) in ensuring laboratory quality, and documents the repeated failure of the Centers for Medicare and Medicaid Services (CMS) to implement this law with respect to genetic testing laboratories. It identifies the lack of transparency regarding laboratory quality as a key impediment to sound healthcare decision making by healthcare providers and patients.
The report also summarizes data from the Genetics and Public Policy Center’s recent survey of genetic testing laboratory directors. Survey findings indicate a clear correlation between participation in proficiency testing, which is not currently required under CLIA for genetic testing, and test quality. The findings also show that nearly three-quarters of laboratory directors surveyed support more oversight of genetic testing under CLIA, and more than 90 percent found proficiency testing to be useful in improving genetic testing quality.
The report concludes that implementation of CLIA with respect to genetic testing laboratories through the creation of a genetic testing specialty is necessary to ensure the quality of genetic testing, to fulfill the promise of genetic medicine, and to protect the public’s health.
Introduction
The Clinical Laboratories Improvement Amendments of 1988 (CLIA) (1) is a littleheralded statute with an important mission. Congress enacted the law out of concern over the poor quality of services being offered by clinical laboratories. Congress wanted to make sure that the millions of tests performed on patients every year provided accurate and reliable results.
Authority for implementing CLIA was delegated to the Centers for Medicare and Medicaid Services (CMS). Thus, while much better-known for its role in administering the Medicare and Medicaid programs, CMS also is responsible for monitoring the quality of nearly 200,000 clinical laboratories in the United States (2), which together perform more than 10 billion tests each year (3).
At the time CLIA was enacted, few human genes had been identified and genetic testing was a nascent field largely confined to esoteric research laboratories or prenatal testing for chromosomal disorders. Not surprisingly, in implementing CLIA CMS focused first on those testing areas that were mature and most in need of strengthened oversight. As a result, CLIA has improved the overall quality of clinical laboratory testing in the United States.
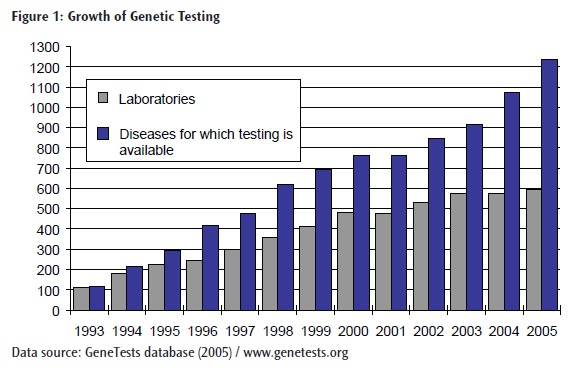
However, in the 18 years since CLIA was enacted and with the completion of the Human Genome Project, genetic testing has moved from the sidelines into mainstream medicine (4). Today there are about 1000 diseases for which genetic tests are available clinically, and several hundreds more are available in a research setting (5) (Figure 1). While initial research focused on rare diseases caused by a mutation in a single gene, more recent research has focused on the identification of genetic contributions to complex, multifactorial conditions such as cancer, diabetes, and heart disease (6, 7).
Read Full Section: Introduction (PDF)
REPORT CONTENT
CLIA: Intent and Implementation
The federal government, through the Department of Health and Human Services (HHS), has been engaged in clinical laboratory oversight for nearly 50 years. Congress enacted the Clinical Laboratories Improvement Act of 1967 in response to reports of high error rates in laboratory testing (16). But the Act was limited in scope, and during the early 1980s Congress again became concerned about laboratory quality. In particular, Congressional hearings revealed that high numbers of false negative results were being reported by laboratories performing Pap smears to screen women for cervical cancer (17). Women with abnormal, possibly cancerous, cells were being incorrectly informed that their Pap smears were normal, leading to needless illness and death.
Congress enacted the Clinical Laboratory Improvement Amendments of 1988, referred to as CLIA, in order to address deficiencies in the original law, and to “strengthen federal oversight of clinical laboratories to assure that the tests results are accurate and reliable.”(17) Congress found that laboratory testing played a critical role in the delivery of health services and in maintaining good health, and that patients both “expect such testing to be done properly” and “assume, quite reasonably, that their interests and the public health are being protected by appropriate government agencies.”(17)
Among the problems uncovered by Congress were a “seriously flawed system” for ensuring laboratory compliance and an “ineffective proficiency-testing system for evaluating the performance of laboratories.” With respect to compliance, Congress found that the government's reliance on private accrediting bodies had created weaknesses in the administration of quality standards, noting that while the government had delegated enforcement to these entities, “these bodies have made plain their preference and capacity is for education, not enforcement.”(17)
Congress noted that proficiency testing “should be the central element in determining a laboratory's competence since it purports to measure actual test outcomes rather than merely gauging the potential for accurate outcomes.”(17) Proficiency testing requires a laboratory to demonstrate that it can obtain the correct answer when performing a test on a tissue sample; thus it serves as a “method of externally validating the level of a laboratory's performance.”(17) But Congress identified serious defects including “lax federal oversight and direction, lack of proficiency testing for many analytes, inconsistent criteria for acceptable laboratory performance, and improprieties by laboratories in handling specimen samples.”(17) Congress intended CLIA to remedy these shortcomings through new, more rigorous laboratory standards (17).
Read Full Section: CLIA: Intent and Implementation (PDF)
CLIA and Genetic Tests
Although Congress was quite clear in the purpose and requirements of CLIA, HHS's implementation of CLIA for genetic testing has been inadequate. Genetic tests are considered high-complexity tests, but no specialty or subspecialty for molecular or biochemical genetics has been established. Thus, there are no specific personnel, quality control, or proficiency-testing requirements for the vast majority of genetic tests. The regulations do include a subspecialty of clinical cytogenetics under the cytology specialty, and establish requirements related to cytogenetics-testing quality control. However, clinical cytogenetics is limited to chromosomal analysis and does not include molecular or biochemical genetic testing. A limited number of proficiency-testing programs exist for molecular and biochemical tests, but enrollment in these programs is not mandated under CLIA. Nor is information about an individual genetic testing laboratory's performance on proficiency testing accessible to the public.
In the absence of a genetic testing specialty for molecular and biochemical genetic testing, laboratories can choose to enroll in other specialties but are not required to. According to results from a recent survey conducted by the Genetics and Public Policy Center, 16 percent of genetic testing laboratories have no specialty certification at all, including a third of high-volume genetic testing laboratories. Among molecular and biochemical genetic testing laboratories with specialty certification, the most common are pathology, chemistry, and clinical cytogenetics (23). However, these specialties have little applicability to a laboratory's proficiency in performing genetic tests. No proficiency-testing programs are mandated for pathology or clinical cytogenetics under current regulations. Moreover, the proficiency-testing programs for chemistry address analytes such as glucose, cholesterol, potassium, and sodium – analytes that are not relevant to assessing proficiency in genetic testing.
Thus in significant ways genetic testing has been left out of CLIA implementation. This situation persists despite the fact that several federal advisory groups have recommended that CMS establish a genetic testing specialty under CLIA (9-11, 53). In 1997, the National Institutes of Health - Department of Energy Task Force on Genetic Testing determined that, in the absence of a genetic testing specialty, “there is no assurance that every laboratory performing genetic tests for clinical purposes meets high standards.”(9) In addition to recommending that a specialty be established, the Task Force also recommended that proficiency testing be mandated for all laboratories doing genetic testing and that a list of laboratories performing genetic tests satisfactorily be made public. In 2000, the Secretary's Advisory Committee on Genetic Testing (SACGT), which succeeded the Task Force, similarly recommended that CLIA regulations be augmented with specific provisions for laboratories conducting genetic tests (10).
Read Full Section: CLIA and Genetic Tests (PDF)
Genetic Testing Laboratory Errors
There is no formal system today for reporting and tracking laboratory errors. The lack of a formal reporting system makes it difficult to detect errors in laboratory testing, and to assess the frequency and consequences of such errors. To some extent, errors in laboratory testing, including genetic testing, are unavoidable, and the goal should be to implement systems designed to reduce errors to the extent feasible and to detect errors when they occur.
Ensuring that genetic testing is optimized to avoid error and that measures are available to detect substandard laboratory performance is of paramount importance. Equally important is providing healthcare providers and patients with sufficient information to assess the quality of genetic testing laboratories they rely on to provide critical healthcare information. Yet CMS has not provided a means for the public to access information about the quality of the laboratories it regulates, or even to determine whether a laboratory is CLIA certified. Although a few studies previously had examined the types of laboratory errors that occur in both genetic (40) and non-genetic testing (41-44) laboratories, or have investigated adherence to professional standards (45), no prior studies had surveyed the practices of genetic testing laboratories or assessed whether the creation of a genetic testing specialty could improve testing quality.
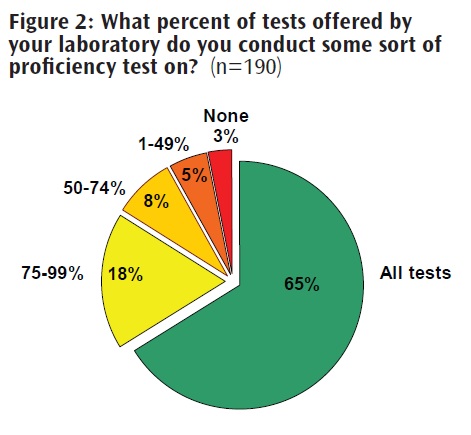
To collect empirical data on laboratory practices and director attitudes regarding oversight, the Genetics and Public Policy Center surveyed 190 directors of molecular and biochemical genetic testing laboratories in the United States (23, 32). The survey sought information about whether laboratories were CLIA certified, were certified in a specialty area, enrolled in formal proficiency-testing programs, engaged in informal proficiency testing when formal programs were not available, had experienced deficiencies in formal proficiency testing, or had reported incorrect test results. The survey also asked what types of errors were most frequently experienced by laboratories, and whether the laboratories complied with specific professional guidelines.
Read Full Section: Genetic Testing Laboratory Errors (PDF)
Opinions on Oversight
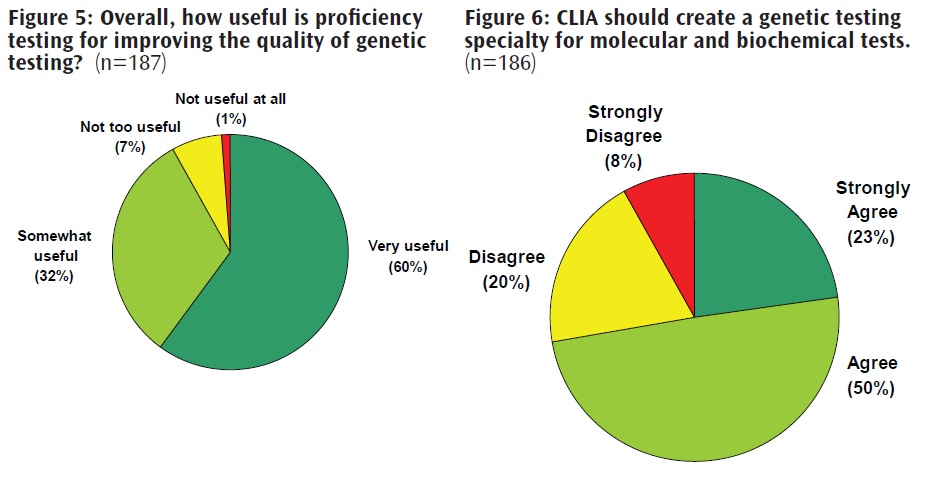
The Genetics and Public Policy Center's survey assessed laboratory directors' attitudes toward laboratory quality and oversight. Nearly all directors found proficiency testing to be very or somewhat useful in improving the quality of genetic testing (23) (Figure 5). A majority (73 percent) of those surveyed agreed or strongly agreed that CLIA should create a genetic testing specialty for molecular and biochemical tests (23) (Figure 6).
While the regulated industry supports the creation of a genetic testing specialty under CLIA, the College of American Pathologists, which accredits clinical laboratories and administers proficiency testing, consistently has opposed the creation of a genetic testing specialty (47-49).
Those who have the most to gain or lose from the accuracy and reliability of genetic testing — that is, patients — resoundingly have expressed their support for the creation of a genetic testing specialty. In February 2006, the Genetic Alliance sent a letter to CMS Administrator Mark McClellan urging him to issue a proposed rule for a genetic testing specialty under CLIA, stating that a specialty “is a necessary first step toward a regulatory system that encourages new technology and ensures safety and accuracy when those technologies are implemented.”(33)
A diverse array of stakeholders also has supported a genetic testing specialty under CLIA. In June 2006, a letter signed by 75 groups comprising patient advocacy organizations, genetic testing laboratories, healthcare provider organizations, and industry urged CMS to issue a proposed rule for a specialty (50). Separately, 14 women's health advocacy organizations also wrote CMS asking for creation of a genetic testing specialty (51).
Conclusion: Why a Specialty is Needed
In the 18 years since Congress enacted CLIA, genetic testing has become a critical part of clinical medicine, and among the fastestgrowing areas of laboratory testing (52). In that time frame, the number of genetic tests has increased more than tenfold. New companies offering genetic tests to healthcare providers and consumers appear with increasing frequency.
Yet, because of CMS's inattention and delay in implementing CLIA, neither healthcare providers nor consumers can be confident in the oversight mechanisms in place to ensure genetic tests are accurate and reliable. While genetic science and genetic technologies have leapt into the 21st century, the agency entrusted with ensuring laboratory quality is stuck in the past. The mandate from Congress under CLIA was clear: Laboratories must participate in proficiency testing for each test they perform unless proficiency testing cannot be developed. Congress was equally clear that the absence of proficiency-testing programs or the difficulty in establishing such programs was not an adequate reason for failing to require participation in proficiency testing. Yet CMS has not mandated participation in proficiency testing for any genetic tests, nor has it demonstrated that creation of proficiency-testing programs is not possible.
Congress was similarly clear regarding the need for transparency regarding laboratory quality. To that end, the law required CMS to create a program to make the results of proficiency-testing programs available to the public. No such program has been created. Nor does CMS make available to the public information on whether a laboratory is certified under CLIA. CMS could easily make this information available to healthcare providers and the public. Without this information, providers and patients are kept in the dark regarding the qualifications and competence of the laboratories that provide critical healthcare information.
To be sure, many genetic testing laboratories in the United States are of very high quality, and go beyond the current minimal standards to ensure the accuracy and reliability of the genetic tests they perform. But, as the Genetics and Public Policy Center's survey of genetic testing laboratory directors reveals, some laboratories are not routinely performing proficiency testing and are not following recommended quality control procedures. Moreover, the survey indicates a correlation between proficiency testing and laboratory quality. A genetic testing specialty under CLIA would provide a mechanism for mandating both formal and informal proficiency testing. Additionally, a genetic testing specialty under CLIA would standardize quality control methods to ensure adherence to the recommended standards.
Genetic testing will have an increasing impact on public health through improved diagnosis, treatment, and prevention of disease. However, the promise of genetics to improve health and healthcare will not be realized unless genetic tests provide accurate and reliable test results. Policy to require that genetic testing be accurate and reliable has not kept pace with the growth of genetic tests. In enacting CLIA, Congress was explicit regarding the need for improved quality standards. With respect to genetic testing quality, CMS has failed to meet the expectations of Congress and the public. The creation of a genetic testing specialty is a critical first step to ensuring that laboratories have demonstrated capability to perform accurate and reliable tests. The time is now for CMS to move expeditiously to protect the public's health.
REFERENCES
1. Public Law 100-578 (1988), codified at U.S. Code, 42, § 263a.
2. U.S. Congress. Senate. Special Committee on Aging. At Home DNA Tests: Marketing Scam or Medical Breakthrough? 109th Cong., 2d sess., 2006 (Testimony of Thomas Hamilton).
3. 2005. How Reliable is Laboratory Testing? American Association for Clinical Chemistry. http://www.labtestsonline.org/understanding/features/reliability.html (accessed August 23, 2006).
4. 2003. International Consortium Completes Human Genome Project. NHGRI. http://www.genome.gov/11006929 (accessed August 23, 2006).
5. GeneTests. University of Washington. www.genetests.org (accessed August 23, 2006).
6. Guttmacher, A. E. and F. S. Collins. 2005. Realizing the promise of genomics in biomedical research.Journal of the American Medical Association 294 (11): 1399-402.
7. Guttmacher, A. E. and F. S. Collins. 2002. Genomic medicine - a primer. The New England Journal of Medicine 347 (19): 1512-20.
8. Marsh, S. and H. L. McLeod. 2006. Pharmacogenomics: From bedside to clinical practice. Human Molecular Genetics 15 (Review Issue 1): R89-R93.
9. Holtzman, Neil and Michael Watson, eds. 1997. Promoting Safe and Effective Genetic Testing in the United States: Final Report of the Task Force on Genetic Testing. NHGRI. http://www.genome.gov/10001733 (accessed August 23, 2006).
10. 2000. Enhancing the Oversight of Genetic Tests: Recommendations of the SACGT. National Institutes of Health. http://www4.od.nih.gov/oba/sacgt/reports/oversight_report.pdf (accessed August 23, 2006).
11. Institute of Medicine. Assessing Genetic Risks: Implications for Health and Social Policy (Lori B. Andrews, Jane E. Fullarton, Neil A. Holtzman, Arno G. Matulsky, eds.). 1994.
12. Clinical Laboratory Improvement Advisory Committee Meeting Summary Reports February 7-8, 2001, January 30-12, 2002, September 11-12, 2002, September 17-18, 2003, February 11-12, 2004, September 22-23, 2004, February 16-17, 2005, September 7-8, 2005, and February 8-9, 2006. http://www.phppo.cdc.gov/dls/cliac/default.asp (accessed August 27, 2006).
13. Federal Register 65 (May 2000): 25928.
14. Federal Register 71 (April 2006): 22595.
15. Meeting between representatives of the Genetics and Public Policy Center and representatives of the Centers for Medicare and Medicaid Services, August 3, 2006.
16. Rivers P. A., et al. 2005. A review and analysis of the Clinical Laboratory Improvement Amendment of 1988: compliance plans and enforcement policy. Health Care Management Review 30 (2): 93-102.
17. House Committee on Energy and Commerce, Clinical Laboratory Improvement Amendments of 1988, 100th Cong., 2nd sess., 1988, H. Rep 100-899.
18. Code of Federal Regulations, title 42, sec. 493. References Public Health at Risk: Failures in Oversight of Genetic Testing Laboratories 21
19. Code of Federal Regulations, title 42, sec. 493.17.
20. Code of Federal Regulations, title 42, sec. 493.15.
21. Code of Federal Regulations, title 42, sec. 493.801.
22. Code of Federal Regulations, title 42, sec. 493.801(a)(2)(ii).
23. Hudson, K., et al. 2006. Oversight of U.S. genetic testing laboratories. Nature Biotechnology 24 (9): 1083-1090.
24. Murphy, Juli, Gail Javitt, and Kathy Hudson. 2005. Creating a Genetic Testing Specialty under CLIA: What are we waiting for? Genetics and Public Policy Center. http://www.dnapolicy.org/resources/McClellanpaper.pdf (accessed August 23, 2006).
25. Javitt, Gail pers. comm. to Judy Yost, July 15, 2005.
26. Yost, Judith pers. comm. to Gail Javitt, September 15, 2005.
27. Hudson, Kathy pers. comm. to Mark McClellan, November 18, 2005.
28. Hamilton, Thomas pers. comm. to Kathy Hudson, Jan. 9, 2006.
29. Testimony of Judith A. Yost, director, Division of Laboratory Services, Centers for Medicare and Medicaid Services, before the Secretary's Advisory Committee on Genetics, Health, and Society, June 26, 2006.
30. 2006. Nutrigenetic Testing: Tests Purchased From Four Websites Mislead Consumers. United States Government Accountability Office. http://www.gao.gov/new.items/d06977t.pdf (accessed August 23, 2006).
31. Federal Register 68 (January 2003): 3639
32. 2006. Practices and Attitudes of Laboratory Directors of Clinical Genetic Testing Laboratories. Johns Hopkins IRB No. NA-00001533. Unpublished data on file with Genetics and Public Policy Center, Washington, D.C.
33. Terry, Sharon pers. comm. to Mark McClellan, February 28, 2006.
34. Smith, Dennis pers. comm. to Sharon Terry, July 17, 2006.
35. Schirmer v. Mt. Auburn Obstetrics and Gynecological Associates, 844 N.E.2d 1160 (2006).
36. Hood v. Lab. Corp. of Am., 2006 U.S. Dist. LEXIS 36464 (D.Md. 2006)
37. Libby, E. N., et al. 2006. False-negative factor v Leiden genetic testing in a patient with recurrent deep vein thrombosis. American Journal of Hematology 81: 284-289.
38. Our stories. Matthew Forbes Romer Foundation. http://www.mfrfoundation.org/stories.php (accessed August 29, 2006).
39. Feiger, J. 2003. Protecting patients while managing lab errors. Perspectives in Genetic Counseling. 25 (3): 4 22 Public Health at Risk: Failures in Oversight of Genetic Testing Laboratories
40. Hofgartner, W.T. and J.T. Tait. 1999. Frequency of problems during clinical molecular genetic testing. American Journal of Clinical Pathology 112: 14-21.
41. Bonini P. et al., 2002. Errors in laboratory medicine. Clinical Chemistry 48 (5): 691-698.
42. Witte, D. L. et al. 1997. Errors, mistakes, blunders, outliers, or unacceptable results: how many? Clinical Chemistry 43 (8): 1352-1356.
43. Howanitz, P. J. 2005. Errors in laboratory medicine: practical lessons to improve patient safety. Archives of Pathology & Laboratory Medicine 129: 1252-1261.
44. Hollensead, S. C., et al. 2004. Errors in pathology and laboratory medicine: consequences and prevention. Journal of Surgical Oncology 88: 161-181.
45. McGovern, M. M., et al. 1999. Quality assurance in molecular genetic testing laboratories. Journal of the American Medical Association 281 (9): 835-840.
46. 2006. Standards and Guidelines for Clinical Genetics Laboratories. American College of Medical Genetics. http://www.acmg.net/Pages/ACMG_Activities/stds-2002/stdsmenu-n.htm (accessed August 23, 2006).
47. Bachner, Paul, pers. comm. to Joe Boone, June 28, 2000.
48. Clinical Laboratory Improvement Advisory Committee Meeting Summary Report, September 10, 1997. http://www.phppo.cdc.gov/dls/cliac/default.asp (accessed August 30, 2006).
49. The Secretary's Advisory Committee on Genetics, Health, and Society's Summary of Third Meeting, March 1-2, 2004, pages 6-7 (public comments of Margaret Gulley, MD, College of American Pathologists). http://www4.od.nih.gov/oba/sacghs/meetings/March2004/SACGHS (accessed August 30, 2006).
50. Terry, Sharon, et al. pers. comm. to Mark McClellan, June 6, 2006.
51. Reproductive Health Technologies Project, et al. pers. comm. to Mark McClellan, July 13, 2006.
52. Frost and Sullivan, U.S. Genetic Diagnostics Markets, Market Report F463-52, 2005.
53. Meeting summaries of the Clinical Laboratory Improvement Advisory Committee. http://www.phppo.cdc.gov/dls/cliac/default.asp (accessed August 30, 2006).
54. Shalala, D. pers. comm. to Ed McCabe, January 19, 2001. http://www4.od.nih.gov/oba/sacgt/McCabe.pdf (accessed August 30, 2006).






