Time For Change
Addressing Conflicts of Interest at Academic Medical Centers
QUICK SUMMARY
Academic medical centers (AMCs) form the intellectual core of medicine, training future doctors and researchers, and establishing standards that guide practicing physicians in the wider community Where pharmaceutical industry marketing conflicts with the goals of patient care and professionalism, AMCs can provide leadership and guidance by establishing new standards on physician-industry relationships
Overview
The pharmaceutical industry spends more than $25 billion each year in direct marketing to physicians, including more than $7 billion for detailing and journal advertising and $18 billion for samples. (1) Ninety thousand sales representatives are deployed.(2) Many promote new and expensive products that lack clear therapeutic advantage and may have unknown adverse effects. (3) Industry representatives often gain access to doctors by offering meals, drug samples and other gifts. This intense marketing is widely believed to undermine quality of care and increase costs to patients, public programs, health care institutions, health insurers and employers. (4-6)
Gifts generate conflicts of interest. Physicians who accept company gifts may feel a need, subconscious or otherwise, to reciprocate. Even small gifts change behavior; (7) public records show that many clinicians receive tens of thousands of dollars per year from the industry. (8)
Industry sales representatives frequently provide inaccurate information (reviewed in Molloy et al.). (9) Yet contact with sales representatives or acceptance of industry support leads to increased prescribing of the funders' products, increased requests for formulary inclusion and decreased use of generic medications. (3, 10) Nearly all physicians (more than 90 percent) have some relationship with industry, (11) but many often fail to realize the extent to which these relationships influence their own prescribing decisions.(12-14)
Relationships between medicine and industry also exist at the institutional level. Many AMCs depend heavily on pharmaceutical company support for research, education and other organizational activities. (15) Positive relationships with industry may confer institutional benefits, but the attendant conflicts of interest must be addressed.
In January 2006, a group of leading physicians and researchers from the Institute of Medicine as a Profession (IMAP) and the American Board of Internal Medicine Foundation called for AMCs to take the lead in ending conflicts of interest between physicians and pharmaceutical companies. Writing in the Journal of the American Medical Association (JAMA), the authors outlined several recommendations toward change. (16) The article generated a great deal of interest in the press and from within the medical profession. (17-22) Following its publication, a number of AMCs strengthened their policies. The Prescription Project was launched by The Pew Charitable Trusts in February 2007 with a major goal of promoting the JAMA recommendations.
To assess current conflict of interest policies at—and recommend “best practices” for— AMCs, the Prescription Project conducted an in-depth investigation of policies, or draft policies, at a number of leading AMCs. Using online research and interviews, we collected information on policies as well as implementation histories, successes, failures, and future plans. Drawing on the JAMA recommendations, we used the following specific criteria for assessing policies:
- Gifting: Do AMCs permit gifts to physicians from industry? Are there any restrictions on “giveaway” items, meals, payment for travel to, or time at, meetings, or payment for CME participation?
- Drug samples: Do AMCs permit physicians to accept samples? Or is there a system (e.g. vouchers for low-income patients) that distances the company from the physician? Are samples limited to patient use, or may physicians use samples for themselves and their families?
- Drug formularies: Do AMCs permit physicians with financial ties to drug companies to serve on committees overseeing formularies or the purchase of medical devices?
- Continuing medical education (CME): How do AMCs manage industry funds for CME? What policies are in place to ensure that CME events remain free of influence from donors?
- Funds for physician travel: Do AMCs permit manufacturers to directly fund travel of faculty and trainees? What policies govern funds for physician travel?
- Speakers bureaus and ghostwriting: Do AMCs allow faculty to serve on speakers bureaus or to publish articles or editorials that are ghostwritten by companies?
- Consulting and research grants: How do AMCs oversee grants for consulting and research? Do they require an explicit contract with specific deliverables? Do they allow “no strings attached” grants and gifts to individual researchers?
In the 18 months since the publication of the JAMA recommendations, more than a dozen AMCs around the country have responded to the call to adopt more stringent regulations.
The Prescription Project has been actively engaged with leaders at AMCs, including the University of California, Los Angeles and the University of Massachusetts, as well as the institutions mentioned in Table 1. We have assisted a number of these centers in drafting and implementing new policies. We are also working with select AMCs to conduct in-depth case studies that will provide new insights for institutions and leaders nationally.
Following are “best policies” in each of the issue areas addressed in the JAMA recommendations. Leaders at other AMCs can use these exemplary policies to begin discussions with faculty and staff about the importance and feasibility of strengthening existing regulations. Doing so will improve patient care and protect the integrity of medical decision-making by reducing the influence of industry marketing on prescribing patterns.
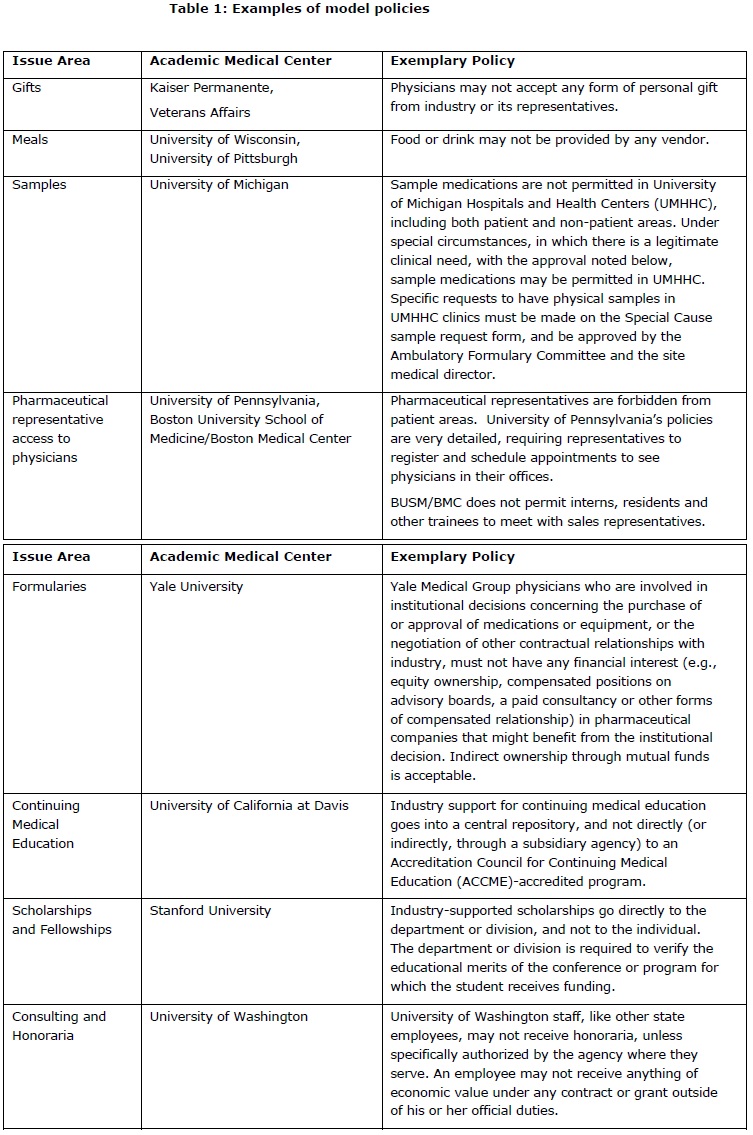
Public pressure for controlling conflicts of interest is growing. The national press reports regularly on the dangers and cost to patients of drug industry influence on physician decisions. Some states, including Vermont, Minnesota and Maine, have already passed laws limiting gifts to physicians or requiring public disclosure, while several Attorneys General have initiated or joined cases against potentially illegal relationships between pharmaceutical companies and physicians. (23-25) Washington, D.C. lawmakers are also considering action. Congressman Peter DeFazio (D-Ore) has introduced a bill requiring drug and device companies to disclose marketing and promotional gifts given to doctors. (26) Senator Chuck Grassley (R-IA) has introduced a similar measure, (27) and the Senate Finance Committee has begun investigating the extent of pharmaceutical industry influence over continuing medical education content. (28, 29) The Senate Aging Committee is also examining the influence of pharmaceutical industry marketing on medicine. (30)
Although some AMCs have begun to address these issues, the national landscape remains relatively unchanged. Through our discussions with AMC leaders, we have identified a number of barriers to moving ahead, and are developing strategies to counter them:
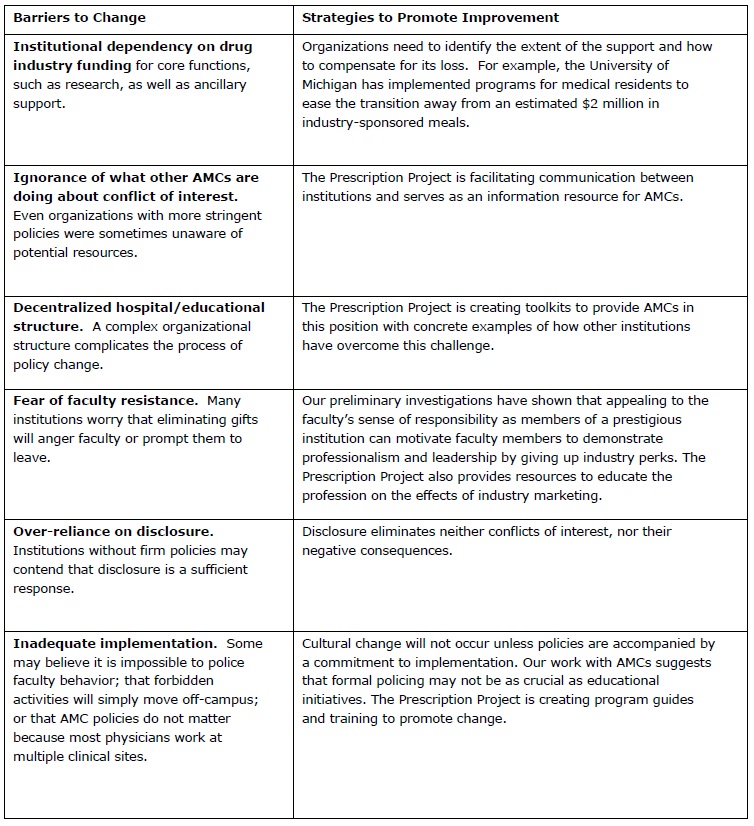
As more AMCs strengthen their guidelines, some of these barriers will disappear. Industry threats to withdraw funding from stringent AMCs will lose effectiveness, and fears of competition for faculty will weaken.
Overcoming barriers and creating momentum for change requires leaders in the profession to think differently about conflict of interest. If the medical profession does not act, it will lose its prerogative.
We urge the Association of American Medical Colleges (AAMC) to provide leadership on these issues across all medical schools and teaching hospitals in the United States. The AAMC has convened a task force dedicated to this issue. To fulfill the AAMC mission of “strengthening the quality of medical education and improving the nation's health by enhancing the effectiveness of academic medicine,” the organization must now promote a national agenda and a level playing field.
The AAMC should:
- Exert leadership by strengthening its guidelines related to conflict of interest
- Provide assistance to member AMCs so they can implement effective reforms
- Create an oversight committee to evaluate the actions of member organizations.
While the AAMC needs to provide overall guidance, it will be individual AMCs that determine the ultimate success or failure of efforts to eliminate conflicts of interest. Therefore, we call on all academic medical centers to:
- Examine best practices at other institutions, including those mentioned
- Assess current policies on conflict of interest
- Engage faculty broadly to build commitment at all levels
- Address key issues and announce the new policies to the professional and broader communities
- Enforce adherence through an effective monitoring system.
Conclusion
The medical profession and the public look to AMCs for leadership. New standards must demonstrate the importance of evidence-based practice, free from industry influence and bias. The Prescription Project is assisting AMCs by facilitating communication, providing toolkits and developing concrete and effective best-practice recommendations.
Strong standards will advance patient well-being and free physicians from conflicts of interest. Now is the time for action.
The Prescription Project (RxP), led by Community Catalyst and the Institute on Medicine as a Profession and funded by The Pew Charitable Trusts, seeks to eliminate conflicts of interest created by industry marketing by promoting policy change among academic medical centers, professional medical societies and public and private payers. The Prescription Project has spent the past six months working closely with academic medical centers to investigate their current polices and promote best practices among them.






