Ensuring the Safety of Compounded Drugs
Study highlights key quality standards
A new study compares safety measures followed by pharmaceutical companies that manufacture drugs with those used by compounding pharmacies, and it highlights the more robust safeguards needed when compounding occurs on a larger scale. Quality Standards for Large-Scale Sterile Compounding Facilities, authored by Clinical IQ LLC and commissioned by The Pew Charitable Trusts, chronicles the changes in scope and magnitude of sterile drug compounding from the 1980s to the present day and the failure of regulatory oversight to keep pace. In addition, it provides essential information for stakeholders and regulators on important quality standards to ensure the safety of large-scale compounding operations.
Traditional drug compounding—the creation of customized medicines to meet a patient's unique needs—has always been a part of pharmacy practice. But dramatic expansion of the sector in recent decades has resulted in production conditions that are on a scale closer to pharmaceutical manufacturing yet without the same oversight or quality standards. In fact, since 2001, over 25 pharmacy compounding errors have been associated with 1,049 adverse events, including 89 deaths.
A nationwide outbreak of fungal meningitis linked to contaminated injections made by a compounding pharmacy in Massachusetts focused national attention on this issue beginning in late 2012, resulting in a renewed state focus on compounding oversight and a 2013 federal law governing compounding. The Drug Quality and Security Act established a new regulatory category for certain pharmaceutical compounders that supply sterile medicines for use in hospitals, doctors' offices, and clinics. These “outsourcing facilities” will be subject to higher quality and safety standards—current good manufacturing practices, or CGMPs—enforced by the Food and Drug Administration. However participation in this new regulatory category is voluntary. Large-scale compounding operations that remain primarily under state oversight must also be held to appropriate quality standards.
The Clinical IQ report calls for meaningful quality standards for all large-scale compounding enterprises—outsourcing facilities and other high-volume compounding pharmacies. Key CGMP provisions critical for outsourcing facilities include:
-
Proper evaluation and qualification of nonsterile starting ingredients. Allowing unevaluated nonsterile ingredients, supplies, and packaging into sterile production immediately introduces variability that may affect drug quality and patient safety. Outsourcing facilities should establish processes to receive, test, and release nonsterile ingredients. Existing standards for sterile compounding do not identify specific requirements for this quality assessment.
-
A robust environmental monitoring system. Medicines must be produced in an environment that controls the risk of contamination and error. All areas of sterile production must be maintained in a strict and sanitary manner, and ongoing and routine sampling must occur to ensure an environmental state of control. The environmental sampling program under existing standards for sterile compounding is inadequate for large-scale operations.
-
Validated production processes. The effectiveness of any procedure used to sterilize a medicine or assure its quality must be established through process validation. Outsourcings facilities must use scientific evidence to validate that a process can consistently deliver a quality product. Quality cannot be adequately assured by product testing alone, which is the present-day method under existing standards for sterile compounding.
Other Differences in Quality Standards
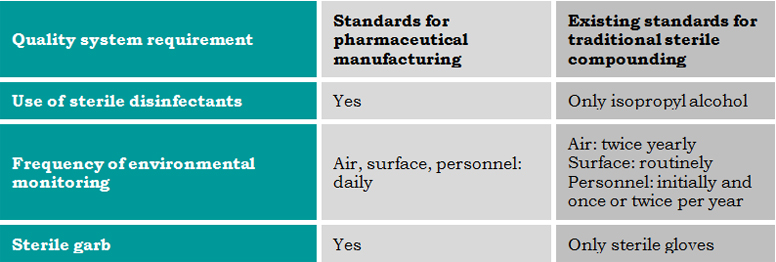 |
Source: E. Kastango and K. Douglass, Quality Standards for Large-Scale Sterile Compounding Facilities (May 2014)






