What Are Compounded Drugs, and How Can They Be Kept Safe?
Drug Quality and Security Act plays key role in important but potentially high-risk aspect of health care
Overview
Pharmaceutical compounding is the creation of medications for patients whose clinical needs cannot be met by commercially available products approved by the Food and Drug Administration. For example, if a patient who cannot swallow pills needs a liquid version of a medicine that is FDA-approved only in pill form, a compounding pharmacy can make the medication.
Pharmacists have always tailored medications to specific clinical situations, and that practice remains an important part of health care. Over time, however, compounding has expanded and evolved. Today, some compounders do not dispense drugs to individual patients but instead produce large quantities for hospitals and other health care providers that need supplies of medications not made by pharmaceutical manufacturers, or not made in the specific form, combination, or strength that patients may require. Examples of these products include epidural analgesics used during childbirth, liquid nutrition for patients who cannot eat and digest normally, and preservative-free versions of sterile products for patient populations such as newborns who may be harmed by certain ingredients that extend a product’s shelf life.
The way compounding is regulated has also changed through history, though sometimes not quickly enough, as was tragically evident in 2012 and 2013. Compounding businesses had dramatically expanded, while oversight did not keep pace. After contaminated compounded products caused a deadly nationwide outbreak of fungal meningitis, lawmakers and regulators enacted federal- and state-level reforms to protect patient safety.
At the federal level, the Drug Quality and Security Act (DQSA), passed on a bipartisan basis in 2013, preserves access to safe compounded medications for patients who need them while helping to protect those patients from the risks of drugs produced under dangerous and illegal conditions. Notably, the law gives FDA the authority to oversee any compounders producing bulk supplies of non-patient-specific drugs and requires those facilities to apply certain protocols to prevent contamination and other potentially dangerous problems.
Distinct safety risks of compounded products
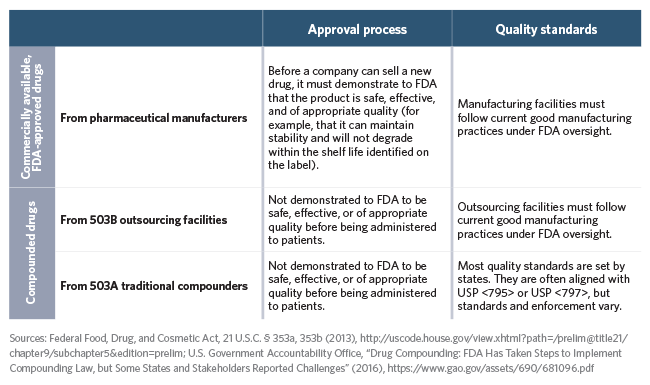
Commercially available, FDA-approved drugs from pharmaceutical manufacturers are characterized by two main patient safeguards that do not apply to most compounded drugs. They must be tested to demonstrate safety and efficacy and go through an approval process before they can be sold, and they must be produced under stringent federal quality standards. The term “quality standards” refers to any requirements for how drugs are manufactured and stored to prevent contamination or other potentially dangerous problems; the specific quality standards for makers of FDA-approved drugs are called current good manufacturing practices (CGMP). They are specifically designed to ensure safety for products produced in batches and stored for relatively lengthy periods of time.
Compared with FDA-approved products, compounded products pose a greater risk, for a few reasons. First, they have not been tested for safety and efficacy, nor have they gone through any approval process, before being dispensed to patients. Moreover, most compounded products come from facilities that do not use the same quality standards as pharmaceutical manufacturers under FDA oversight.
Which companies and individuals can compound drugs?
In general, businesses that produce commercially available medications must obtain FDA approval and adhere to the federal labeling and quality requirements that apply to manufactured drugs. Compounders—businesses or individuals making customized drugs that have not been approved by FDA—are exempt from certain laws that would otherwise apply. The DQSA amended the Federal Food, Drug, and Cosmetic Act (FDCA) to recognize two categories of drugs that can be made outside of FDA’s approval processes:
- Patient-specific compounded drugs made by traditional compounders:
- Medications made by traditional compounders are tailored to individual patients. Traditional compounders include pharmacists practicing in a variety of settings such as community pharmacies and hospitals, as well as physicians who create drugs for administration to their patients.
- Section 503A of the FDCA establishes certain requirements for drugs compounded by traditional compounders, including that they be made only from permitted ingredients and dispensed only upon receiving a prescription or medical order for an identified individual patient.
- Stock supplies of compounded drugs made by outsourcing facilities:
- Outsourcing facilities produce bulk supplies of compounded drugs for hospitals, doctors’ offices, and other health care facilities.
- Section 503B of the FDCA requires that outsourcing facilities register with FDA, comply with FDA’s CGMP quality standards, adhere to specific labeling requirements, and report adverse events—instances of medically undesirable experiences associated with the use of a drug. Because they are subject to increased federal quality standards, drugs made by outsourcing facilities may be sold to providers without first receiving a prescription.
If a company does not have FDA approval for a product, and does not fit into either of the above categories, it is selling an unapproved new drug in violation of federal law.
Which drugs can be compounded?
Neither FDA nor state authorities evaluate compounded drugs for safety or efficacy, but compounders cannot just use any chemical to create a new drug. Instead, the FDCA defines the particular active drug ingredients— known as bulk drug substances—that can be used to create compounded drugs.
Under Section 503A of the FDCA, traditional compounders can make drugs using only bulk drug substances that fit into one or more of the following categories:
- Drug substances for which the United States Pharmacopeia (USP) has set standards for strength, quality, and purity in a monograph.
- Drug substances that are part of FDA-approved products.
- Other drug substances that are on a list of ingredients FDA creates by regulation.
Under Section 503B of the FDCA, outsourcing facilities can use only ingredients found on either of two lists that FDA maintains: one identifying bulk drug substances for which there is a clinical need that cannot be met by existing FDA-approved products, and the other identifying FDA-approved drugs that are currently in shortage, which compounders are allowed to copy.
The law also requires that bulk drug substances used to compound drugs comply with the USP standards for the drug (if there are such standards). The bulk drug substance must also have been manufactured at an establishment that is registered with FDA and subject to FDA inspection.
Even though compounders can use ingredients that are components of FDA-approved drugs, they generally cannot create large quantities of products that are essentially a copy of approved products (with the important exception for compounders making copies of drugs that are on the FDA shortage list). These provisions of the law preserve FDA’s approval process for drug companies, which is critical to protecting patient safety, while allowing compounders to fill the gap when no approved drug is available to meet a patient’s clinical needs.
These statutory restrictions on bulk drug ingredients and copying approved products help ensure that compounding does not undermine the protections of the drug approval system. Some compounders that have copied FDA-approved drugs, made medications that differ only slightly from such products, or made entirely new products from bulk ingredients may assert that their products are less expensive and, in some cases, actually better than FDA-approved products. However, since compounded medications do not undergo safety and efficacy testing before being dispensed to patients, these drugs have not actually been shown to be safe and effective.
What quality standards are required for compounded drugs?
To avoid patient harm, compounded drugs must be free from contamination and must include the correct ingredients in the right amounts. Safety depends on whether compounders follow appropriate quality standards.
Different types of compounding carry different risks if something goes wrong. For example, products that are injected are more likely to cause an infection if they are contaminated than are products in other dosage forms. Medications made in bulk have the potential to harm more people if a mistake is made in their preparation. Corresponding sets of quality standards are designed to mitigate those varying safety risks:
- For pharmacies that dispense drugs one prescription at a time under 503A, the USP developed standards that guide pharmacists on how to minimize risk when creating compounded drugs. The USP’s sets of standards for compounders include:
- USP <797>, which applies to sterile compounding (the creation of products that are required to be sterile because, for example, they are administered to patients via injection or administered directly to an area highly susceptible to infection, such as the eye, bladder, or lungs).
- USP <795>, which applies to nonsterile compounding (the creation of products administered in other dosage forms, including, for example, most pills, capsules, and topical creams).
Most states require compounders to follow the USP’s standards. Even in states without those requirements, FDA has outlined minimum standards for ensuring that all drugs—no matter where they are made—are produced under sanitary conditions.
- For outsourcing facilities that produce compounded products under 503B, FDA requires quality standards similar to the CGMP that apply to drug manufacturers.
Approval and Quality Requirements for Drugs
A comparison of drugs made by pharmaceutical manufacturers, outsourcing facilities, and traditional compounders:
What can happen when appropriate quality standards are not followed?
In 2012 and 2013, more than 750 people became ill and more than 60 died in a fungal meningitis outbreak that occurred when contaminated drugs were injected into their spines and joints. Many survivors still struggle with chronic, and in some cases disabling, health problems. The drugs were traced to a Massachusetts pharmacy that shipped tainted injections to more than 20 states.
The outbreak drew public and policymaker attention to the risks of drug compounding and brought to light weaknesses in state and federal oversight, prompting reforms at both levels. Congress enacted federal reforms through the bipartisan DQSA. Many states also implemented reforms of their own, such as requiring stateregulated compounders to follow USP quality standards.
While the meningitis outbreak is the most extensive known example of harm to patients from compounded drugs, there have been many other cases of serious illness, injury, and death associated with such medications. Adverse events usually result from contamination but also can be caused by other errors, such as a drug being overly potent to the point of toxicity. Quality standards are designed to prevent problems from occurring and, when they do, to detect them before products reach patients.
Why is the DQSA’s prescription requirement for traditional compounders critical for patient safety?
Significantly, the DQSA created a category of businesses that can compound and distribute drugs without first receiving a prescription for an identified individual patient: outsourcing facilities. To qualify for this category, a business must register with FDA and adhere to federal quality standards, CGMP requirements, and certain other conditions. Traditional compounders that do not meet these criteria must receive a prescription before dispensing a drug, a requirement that is consistent with a criterion that Congress first adopted in 1997 to protect patients from drug compounding risks.
To ensure that facilities producing drugs in bulk supplies are overseen under the appropriate regulatory scheme, there must be a clear line distinguishing traditional compounders from outsourcing facilities. That line is the prescription requirement that applies to traditional compounders under the federal DQSA.
If traditional compounders could dispense drugs without prescriptions, there would be no inherent check on their production scale, allowing them to produce bulk quantities and leading back to serious safety threats that Congress worked to solve in creating the distinct categories. The prescription requirement ensures that stock supplies of drugs, which pose a greater safety risk because of their typically longer shelf life and larger batch sizes, are subject to the quality standards appropriate to bulk production and longer shelf life.
Conclusion
To protect public health, it is important to preserve access to safe compounded medications for people who need them, and to effectively implement and enforce the DQSA. If this law is weakened—by eliminating or modifying the prescription requirement for traditional compounders, for instance—it will only be a matter of time before drugs that have been compounded or stored under dangerous conditions are responsible for another widespread and deadly crisis.