Federal Agencies Have Substantial Authority to Boost Methadone Access
Study finds government can make key addiction treatment more available, even without Congress
Methadone, a Food and Drug Administration-approved treatment for opioid use disorder (OUD), can reduce illicit opioid use and prevent overdose deaths, which reached record numbers last year. More than 400,000 people in the United States received methadone as part of their addiction treatment in 2019, and many more could benefit from this lifesaving medication—an estimated 1.6 million people had OUD that same year. But federal regulations limit the availability of methadone at a time when there is a great need to boost access to this effective treatment.
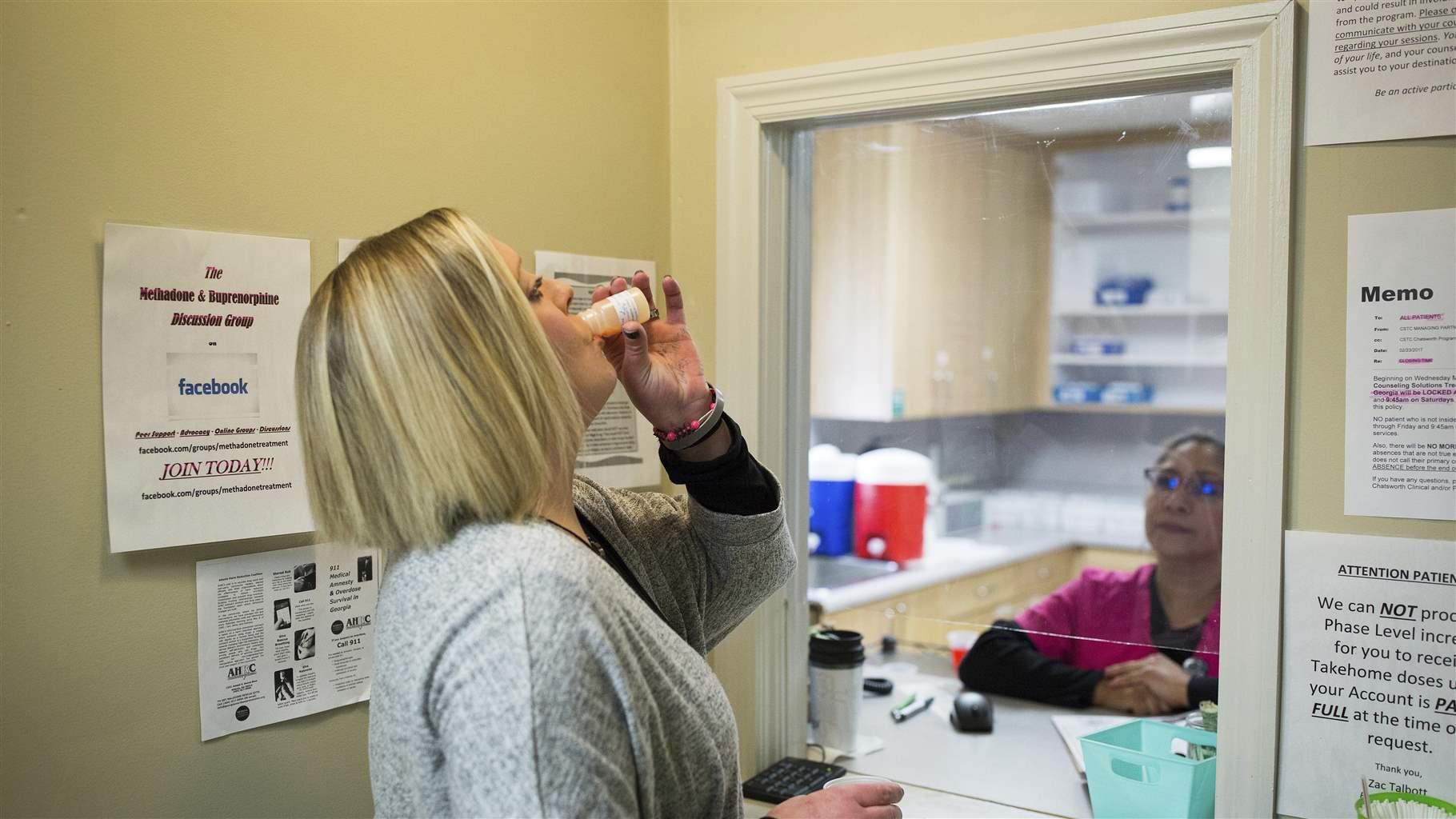
Today, methadone for OUD can be dispensed only at licensed opioid treatment programs (OTPs), which are specialized health care facilities subject to different criteria than other medical offices. The practitioners who work there also must meet stringent federal and state requirements. In addition, under current rules, people being treated with methadone often must go to the OTP almost daily to receive their dose. The sometimes-lengthy trips then can interfere with patients’ abilities to work and live their lives.
To improve access to OUD treatment, the Biden administration must consider ways to remove restrictions without relying on legislative action by Congress. A new report from the Regulatory Studies Center at George Washington University (GWU), published Aug. 11 and funded by The Pew Charitable Trusts, finds that the regulatory barriers to accessing methadone can be removed or amended by executive branch agencies—specifically, the Substance Abuse and Mental Health Services Administration (SAMHSA) and Drug Enforcement Administration.
What could change
SAMHSA and DEA currently have restrictions and regulations in place that can, and should, be changed to broaden methadone access.
First, SAMHSA now requires OTPs to abide by restrictions to get and maintain DEA certification to run as narcotic treatment programs. Many of these rules could impede access to methadone; for example, patients must be addicted to an opioid for at least one year prior to admission, drug testing must be performed for each patient, ancillary services such as counseling must be offered, and patients who do not meet specified take-home criteria (e.g., length of time in comprehensive maintenance treatment) must make almost daily visits to the facility to receive treatment. Studies have found that such policies can reduce adherence and impede access to treatment.
SAMHSA has the legal authority to remove or amend such restrictions through guidance or the rule-making process, according to the GWU study. The latter would require building an administrative record to support the changes, including the evidence behind them, and a consultation with the U.S. attorney general. Issuing guidance would be a quicker and less cumbersome process. The agency used this approach to loosen restrictions during the COVID-19 public health emergency, but these changes would be less permanent than through rule-making and subject to the discretion of agency officials.
Second, the DEA regulations include a burdensome requirement that methadone for OUD can be dispensed only at an OTP and may not be prescribed for pickup at a pharmacy. Because the number of pharmacies far exceeds the number of OTPs, pharmacy dispensation of methadone could greatly increase treatment access.
DEA could change this without additional authorization from Congress because the agency’s regulation on dispensing methadone is more restrictive than current law, according to the report.
Third, DEA could change methadone from a Schedule II controlled substance to Schedule III, which would allow health practitioners to prescribe or dispense the medication for OUD without a special DEA or SAMHSA registration. This action by DEA would bring methadone into line with other drugs used to treat the disorder, such as buprenorphine. Specialized training and certification are still required to prescribe this medication. If methadone was a Schedule III controlled substance, practitioners who have already obtained a waiver to treat patients with buprenorphine would be permitted to prescribe methadone as well. Such a change could encourage more providers to obtain a waiver.
To do this, DEA would have to work with the federal Department of Health and Human Services (HHS) to build an administrative record to support such a move. This would entail a “scientific and medical evaluation” of the substance from FDA and scheduling recommendations from the HHS assistant secretary for health to DEA.
Finally, DEA and SAMHSA could eliminate or streamline other lower-level barriers that collectively may play a role in making it difficult and costly for OTPs to operate, therefore reducing methadone access. These include:
- The DEA requirement that each practitioner, hospital, or retail pharmacy that prescribes or dispenses a controlled substance must be registered with the agency and obtain a separate registration as a “narcotic treatment program.” Stand-alone opioid treatment programs are the only health care facilities that must register separately with DEA as narcotic treatment programs.
- SAMHSA’s cumbersome certification and accreditation process for OTPs. Agency permission for a facility to operate as an OTP expires, or must be renewed, after a maximum of three years. Program applications must include a variety of information, including a “description of the organizational structure” of the OTP and their sources of funding. Following this process, SAMHSA has to complete a consultation with the relevant state agency that oversees OTPs.
- DEA’s extensive security controls for controlled substances. Programs must keep methadone in a locked steel cabinet or vault to DEA’s specifications, and depending on the weight of the cabinet and quantity of methadone, they must be bolted or cemented to the floor and have an alarm system. Registrants also must notify DEA of theft and “significant loss” of methadone—there are specific criteria for determining whether a loss is significant, including quantity lost, whether the loss can be attributed to specific people or activities, and patterns of losses over time and local trends. Only a licensed practitioner or an authorized individual can sign an invoice for the controlled substances that the program receives, and patients must wait in a separate area from the narcotic storage area.
- DEA’s requirement that registrants keep detailed methadone inventories and records as well as a dispensing log that tracks the amount of medication dispensed or administered to a patient. OTPs that are permitted to use a computer for data storage must have a system preapproved by DEA and still produce signed hard copies of each day’s dispensing log.
These may not be the largest barriers identified by public health experts and advocates, but appropriate updates and changes could help boost the supply of providers available to care for patients as the nation faces a still-growing opioid crisis. All policies at OTPs should serve the public interest and not needlessly impede treatment access.
The GWU report identifies opportunities for SAMHSA and DEA to update their policies around methadone dispensation. Doing so would be an important step forward in improving the treatment landscape for OUD patients.
Sheri Doyle manages The Pew Charitable Trusts’ substance use prevention and treatment initiative.