Health Experts Establish Targets to Improve Hospital Antibiotic Prescribing
National data shows inappropriate prescribing, opportunities for improvements
This brief was updated on March 19, 2021 to correct statistics on fluoroquinolone prescriptions.
Overview
According to the Centers for Disease Control and Prevention, at least 2.8 million antibiotic-resistant infections occur each year, killing at least 35,000 people. All antibiotic use contributes to resistance. To slow the rise of this growing public health threat, antibiotics should be used only when needed and when prescribed appropriately: the proper drug at the correct dose for the right duration. Minimizing inappropriate antibiotic use in hospitals is a vital element in the fight against antibiotic resistance because more than half of patients admitted to hospitals will receive these drugs. Determining how much antibiotic prescribing is inappropriate and setting national targets to reduce such use are necessary steps for guiding clinical efforts and policies that promote improved antibiotic use.
Beginning in 2018, The Pew Charitable Trusts partnered with the CDC and other public health and medical experts to evaluate antibiotic use in hospitals and set national targets to improve prescribing. Because of the complexity and diversity of illnesses among hospitalized patients, and the limitations on available clinical data for all antibiotic use in hospitals, the panel focused its analysis on four categories of prescribing that account for the most common antibiotic therapies in U.S. hospitals. Using national prescribing data, the experts examined the use of two types of antibiotics—vancomycin and fluoroquinolones—and antibiotic treatments associated with two conditions: community-acquired pneumonia (CAP) and urinary tract infections (UTIs).
Key findings from the analysis showed ample room for improvement:
- Nearly 56% of the prescriptions examined were inappropriate in terms of the specific antibiotic prescribed, the duration of treatment, or the illnesses for which they were given.
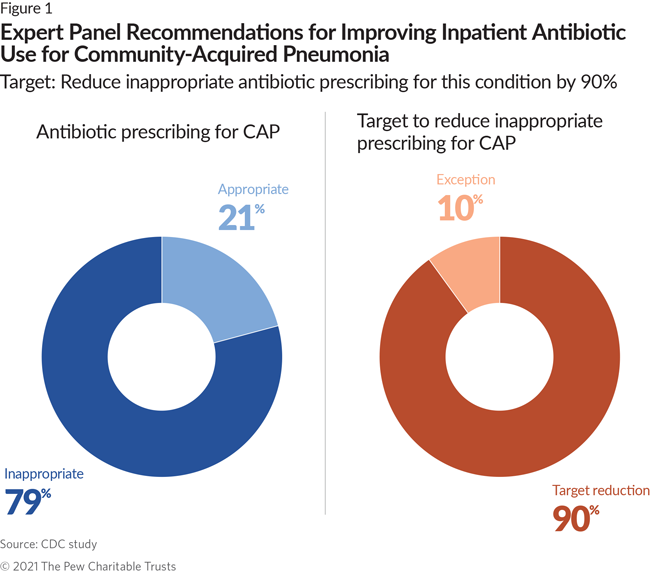
- 79% of all antibiotic prescriptions for CAP and 77% of those for UTIs were inappropriate. The panel recommended a national target to reduce inappropriate use for each of the conditions by 90%.
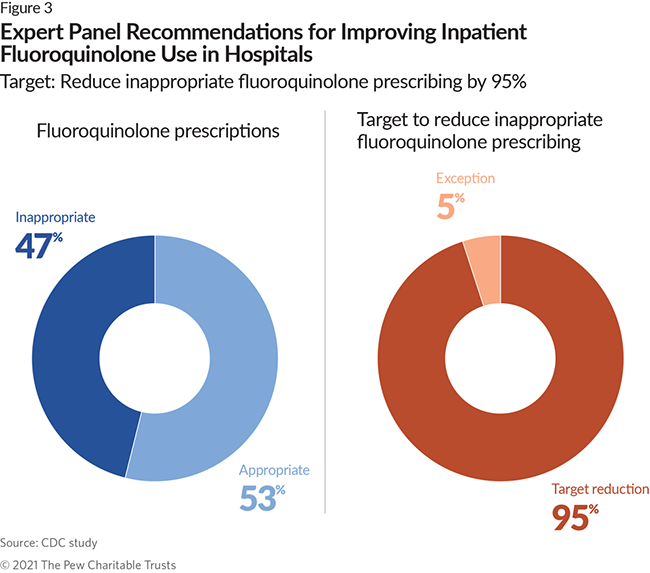
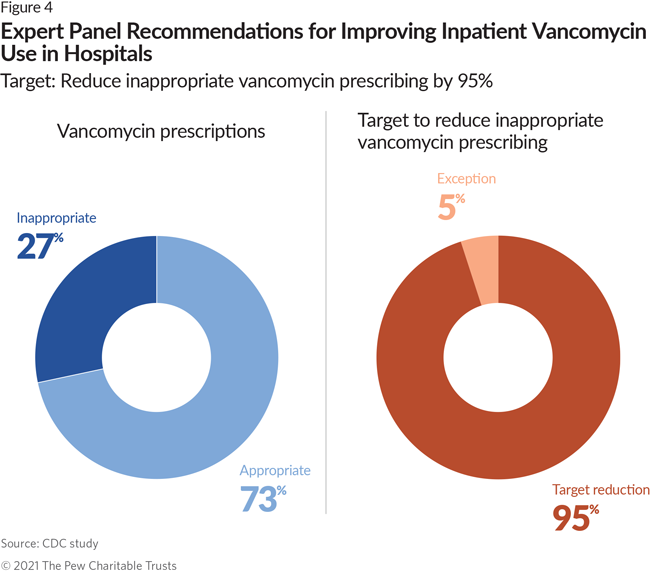
- 47% of all fluoroquinolone and 27% of all vancomycin prescriptions were inappropriate. The panel recommended a national target to reduce inappropriate use for each of the antibiotics by 95%.
Meeting these national reduction targets will require widespread adoption of effective antibiotic stewardship programs (ASPs), which promote responsible antibiotic prescribing, in order to minimize the harmful effects of inappropriate or unnecessary antibiotic use for patients and slow the spread of resistance. Building on the federal policy that requires stewardship programs in most hospitals,1 policymakers, public health agencies including state health departments, private and public payers, hospitals, and health systems still need to take additional steps to ensure that ASPs are as effective as possible:
- Existing stewardship policies and programs should emphasize reducing inappropriate antibiotic prescriptions for CAP and UTIs and inappropriate use of fluoroquinolones and vancomycin.
- Hospitals should report antibiotic use to the CDC’s National Healthcare Safety Network (NHSN) Antimicrobial Use Option to track use and measure the impact of stewardship efforts.
- Existing quality-based reimbursement programs from private and public insurers should incentivize hospitals to implement high-quality ASPs.
- Health care facilities, particularly small and critical access hospitals, need technical assistance and financial support to improve ASP implementation.
Antibiotic Use in Hospitals
In U.S. hospitals, nearly 60% of patients receive antibiotics during their stays.2 Given the volume of inpatient prescribing, it is critical that hospitals minimize inappropriate antibiotic use. In addition to contributing to resistance, the overuse of antibiotics can lead to adverse events such as allergic reactions, which can range from minor rashes to life-threatening illness including Clostridioides difficile (C. difficile) infections, formerly known as Clostridium difficile.
National targets to reduce inappropriate prescribing
In 2018, The Pew Charitable Trusts convened a panel to assess the appropriateness of current antibiotic use in hospitals and establish national data-driven targets to reduce inappropriate prescribing. The panelists, who included CDC experts and other public health and medical experts, represented a wide range of specialties and were selected based on their expertise in inpatient antibiotic prescribing. (See Appendix B.)
The CDC experts used national data collected by the CDC’s Emerging Infections Program (EIP) surveillance network to identify which infections are most commonly associated with antibiotic prescribing across a wide range of hospitals and which antibiotics are the most commonly prescribed. They found that two conditions (CAP and UTIs) and two agents—fluoroquinolones and vancomycin—together account for the most common inpatient prescribing.
Incorporating feedback from the expert panel, CDC experts examined clinical and laboratory data to measure the extent to which antibiotic prescribing for the two conditions and two agents aligned with established treatment guidelines. The panelists identified specific evidence-based exceptions where providers might deviate from treatment guidelines—for example, when a secondary infection is present. Based on the expected prevalence of such exceptions, the panel estimated how much antibiotic use could be eliminated for each condition and agent. (See Appendix C for more information about the methodology.)
Community-acquired pneumonia
CAP is a respiratory infection, and common symptoms include fever, cough, and difficulty breathing. This illness is a leading cause of death in the U.S.3 About 1 million people are hospitalized and approximately 50,000 people die as a result of pneumonia each year in the U.S.4
Current national treatment guidelines for CAP define the types of antibiotics that are appropriate and effective for treating this condition and recommend that they not typically be used for more than seven days.5 Based on these guidelines, the CDC experts measured how frequently nonrecommended antibiotics were used and how often the recommended duration of treatment was exceeded. They determined that 79% of antibiotic prescribing to treat CAP is inappropriate. Almost 60% of the inappropriate prescribing is attributed to exceeding the recommended seven days of treatment, and use of the wrong antibiotic accounts for most of the remaining inappropriate cases.
The expert panel concluded that medically justifiable exceptions to the treatment guidelines occur in about 10% of all CAP cases—such as when a patient has a particularly severe CAP infection or may need additional antibiotic therapies because of secondary complications. The experts therefore determined that 90% of the inappropriate use should be eliminated. They further recommended that stewardship interventions in this area focus on ensuring the appropriate duration of treatment.
Urinary tract infections
A UTI is a common infection of the bladder, urethra, or ureter and is one of the most common reasons that individuals are prescribed antibiotics.6 Many UTIs are mild and can be treated in outpatient settings. However, severe UTIs can lead to kidney infection, a serious illness that often requires hospitalization. Certain populations, such as the elderly, are also at high risk of hospitalization for UTIs.
Established treatment guidelines support antibiotic treatment for UTIs when an infection is confirmed by laboratory testing and specify which antibiotics should be used and for how long.7 The CDC experts measured how frequently inpatient antibiotic prescribing for UTIs that were present when a patient was admitted to a hospital deviated from the recommended duration of treatment, and whether antibiotics should have been prescribed in the first place. They found that in 77% of cases, prescribing for UTIs was inappropriate. In most instances where antibiotic use was not supported, the antibiotics were prescribed to patients who lacked symptoms or microbiology test results consistent with UTIs. The experts estimated that in about 10% of UTI cases, circumstances such as secondary complications or severe infections may allow for exceptions to the treatment recommendations. The panel therefore recommended a national target to reduce inappropriate UTI prescribing by 90%.
Fluoroquinolones
Fluoroquinolones are broad-spectrum antibiotics most commonly used to treat Gram-negative bacterial infections and are one of the most commonly prescribed classes of antibiotics in hospitals.8 Notably, these antibiotics can have serious side effects, including tendon rupture and damage to the central nervous system.9 Given these dangers, the U.S. Food and Drug Administration recommends that fluoroquinolones not be used to treat certain uncomplicated bacterial infections such as urinary tract and sinus infections.10 In 2018, the FDA issued an additional warning about the risks associated with fluoroquinolones and stressed that they should be used only when the benefits outweigh the risks.11
The CDC experts analyzed the frequency of fluoroquinolone prescribing in cases where such prescribing is not recommended, as well as treatments that exceeded recommended durations. They determined that 47% of fluoroquinolone use is inappropriate. The expert panel set a target of a 95% reduction in this use, which allows room for rare exception events. However, the panel agreed that these estimates were conservative because of the limitations in the clinical data available for this analysis and that there are probably opportunities to reduce fluoroquinolone use beyond what was identified in this project. Given the high risks of toxicity and adverse events associated with fluoroquinolone use, the panel recommended that alternative and equally effective antibiotic agents always be favored over fluoroquinolones when available.
Vancomycin
Vancomycin is an antibiotic that targets resistant Gram-positive bacteria. It is often used to treat infections caused by methicillin-resistant Staphylococcus aureus. Like fluoroquinolones, vancomycin is one of the most frequently prescribed antibiotics in inpatient settings, and its use has increased significantly.12 Like any medication, vancomycin treatment carries certain risks to patients; for instance, it has been associated with kidney damage.13 This medication should be used only when necessary, and treatment guidelines recommend close monitoring of dosing to avoid dangerous side effects.14
National treatment guidelines specify the conditions for which vancomycin use is appropriate and provide guidance on dosing and monitoring.15 Using these guidelines, the CDC experts determined the frequency of vancomycin therapies used for conditions where the antibiotic was not indicated, and how often vancomycin therapy use was inappropriately continued despite microbiology laboratory data supporting its discontinuation. Based on this analysis, they found that 27% of such use in inpatient settings is inappropriate. The panel found that this unsupported use should be reduced by 95%.
Considerations for pediatric populations
The expert panel determined that modifications to the current UTIs and CAP would be needed for it to be more applicable to pediatric populations because of differences in diagnostic criteria or recommended first-line therapies. The panel determined that the methodology used to assess fluoroquinolone use in adults could be applied to pediatric populations, with the understanding that fluoroquinolone use is significantly less frequent in pediatrics. The experts did not come to a consensus on whether the methodology for vancomycin could be applied to pediatric populations, given the significant differences in indications for use.
Reaching national targets for improved antibiotic use: the role of antibiotic stewardship
Antibiotic Stewardship Programs
Antibiotic stewardship programs are designed to ensure that antibiotics are given only when necessary and that they are given appropriately when needed. Such programs improve patient outcomes, reduce health care costs, and minimize the threat of antibiotic resistance.16
Meeting the national targets for the two conditions and two agents will have a significant impact on improving overall antibiotic prescribing in U.S. hospitals. But it will require countrywide implementation of high-quality ASPs.
Inpatient antibiotic stewardship: What does it look like?
There is no one-size-fits-all approach to antibiotic stewardship. Hospitals vary in terms of size, patient population, needs, and resources. To address these differences, the CDC created two guides, “The Core Elements of Hospital Antibiotic Stewardship Programs” and “Implementation of Antibiotic Stewardship Core Elements at Small and Critical Access Hospitals,” that describe a wide variety of interventions that hospitals can tailor to meet their own needs. The strategies outlined in these guides can help improve prescribing, but alone they are not enough to achieve widespread, effective stewardship efforts in hospitals. To accomplish this goal, a number of policies and activities must be prioritized by a range of stakeholders. For example:
- Building on recent regulatory requirements to ensure that all hospitals have effective, high-quality stewardship programs with activities designed to reduce inappropriate antibiotic use. Public agencies and nongovernmental organizations that oversee quality of care—such as the Centers for Medicare & Medicaid Services (CMS) and The Joint Commission—can create or refine policies that require and establish specific standards for stewardship activities with an emphasis on reducing inappropriate use for the two agents and conditions assessed.
- Incentivizing hospitals to report their antibiotic use in the CDC’s National Healthcare Safety Network Antimicrobial Use Option to track use and measure the impact of stewardship efforts. The CDC, state departments of health, and hospital associations can help prioritize and support the widespread uptake of antibiotic use reporting into the NHSN, the nation’s most widely used health care-associated infection surveillance network. In NHSN, hospitals can track and report their antibiotic use, compare their use against that of other hospitals, and detect areas where interventions are needed to reduce inappropriate prescribing. Widespread antibiotic use reporting into NHSN allows public health agencies to identify and monitor inappropriate prescribing patterns at the national, state, and local levels; develop strategies to improve prescribing and combat resistance; and measure the progress and efficacy of implemented activities and policies.
- Using existing payer-based, quality-based reimbursement programs to incentivize hospitals to implement high-quality ASPs. CMS and health plans can use existing quality-based reimbursement programs to encourage hospitals to improve the quality of their ASPs.
- Providing technical assistance and financial support to facilities, particularly small and critical access hospitals, to support ASP implementation. The CDC, state and local health agencies, health systems, and quality improvement organizations can support facilities, identify targets for stewardship, and provide the resources necessary to implement them. Professional societies can develop new and update existing clinical and stewardship guidelines that define appropriate antibiotic use.
Conclusion
The targets outlined in this report are ambitious. To achieve these goals, stakeholders across the country must prioritize improved antibiotic prescribing through sustained antibiotic stewardship. They must commit to reducing unnecessary antibiotic use through meaningful ASPs in hospitals to improve patient safety and reduce the threat of antibiotic resistance.
Appendix A: Glossary
- Broad-spectrum antibiotics: antibiotics that are effective against a wide range of infectious microorganisms.17
- Clostridioides difficile (formerly known as Clostridium difficile): a bacterium that causes diarrhea and colitis and poses significant risk to patients during or following antibiotic use.18
- Community-acquired infection: an infection contracted outside a health care setting.19
- Culture: a laboratory test used to confirm infection.20
- Fluoroquinolones: a group of broad-spectrum antibiotics most commonly used to treat Gram-negative bacterial infections.21
- Methicillin-resistant Staphylococcus aureus: a type of bacteria that is resistant to penicillin-related antibiotics.22
- National Healthcare Safety Network: a health care-associated infection tracking system that provides facilities, states, regions, and the nation with data needed to identify problem areas, measure the progress of prevention efforts, and ultimately eliminate health care-associated infections.23
- Pneumonia: acute inflammation of lung tissue resulting from an infection.24
- Urinary tract infection: infection of the kidneys, bladder, urethra, or ureter.25
- Vancomycin: an antibiotic used to treat Gram-positive bacterial infections.26
Appendix B: Expert panel members
| Name | Affiliation | Specialty |
|---|---|---|
| Denise Cardo | Centers for Disease Control and Prevention | Public health, infectious diseases |
| Susan Davis | Wayne State University; Henry Ford Hospital | Pharmacy, infectious diseases |
| Elizabeth Dodds Ashley | Duke University | Pharmacy, infectious diseases |
| Scott Fridkin | Emory University | Epidemiology, infectious diseases |
| Matthew Bidwell Goetz | VA Greater Los Angeles Healthcare System; David Geffen School of Medicine at University of California, Los Angeles | Infectious diseases |
| Kalpana Gupta | VA Boston Healthcare System; Boston University School of Medicine | Infectious diseases, public health, epidemiology |
| Lauri Hicks | CDC | Public health, infectious diseases |
| David Hyun | The Pew Charitable Trusts | Pediatric infectious diseases |
| Timothy Jenkins | Denver Health | Infectious diseases |
| Makoto Jones | VA Salt Lake City Health System | Epidemiology, infectious diseases |
| Shelley Magill | CDC | Public health, infectious diseases |
| Holly Maples | University of Arkansas for Medical Sciences College of Pharmacy; Arkansas Children’s Hospital | Pediatric clinical pharmacology, infectious diseases |
| Larissa May | University of California, Davis | Emergency medicine |
| Talene Metjian | Children’s Hospital of Philadelphia | Antimicrobial stewardship, pediatric infectious diseases |
| Joshua Metlay | Massachusetts General Hospital | Internal medicine, epidemiology |
| Marc Meyer | Southwest Health System | Infection control, antibiotic stewardship |
| Melinda Neuhauser | CDC | Pharmacy, public health, infectious diseases |
| Jason Newland | Washington University in St. Louis | Pediatric infectious diseases |
| Erin O’Leary | CDC; Lantana Consulting Group | Public health, statistics |
| Arjun Srinivasan | CDC | Public health, infectious diseases |
| Pranita Tamma | Johns Hopkins University School of Medicine | Pediatric infectious diseases |
| Barbara Trautner | Baylor College of Medicine | Infectious diseases, internal medicine |
| Valerie Vaughn | University of Michigan; VA Ann Arbor Healthcare System | Hospital medicine |
Appendix C: Data sources and methodology for assessing inpatient antibiotic use
The targets outlined in this report are based on data collected by the CDC’s Emerging Infections Program (EIP) through its Healthcare-Associated Infections Community Interface (HAIC) activity. The EIP—a network of 10 state health departments and their academic and other partners—conducts population-based surveillance of health care-associated infections and antimicrobial-resistant pathogens. It also undertakes special projects to define the epidemiology of health care-associated infections and antimicrobial use in hospitals and nursing homes.
The EIP’s HAIC began collecting data on antimicrobial use in hospitals in 2011. In collaboration with the EIP network, the CDC conducted a survey to measure the amount of antibiotic use in hospitals. This survey found that about half of all patients were receiving one or more antibiotics and that use of broad-spectrum antibiotics was high.27 The results suggested that there is room to reduce antibiotic use in inpatient settings. However, the survey did not assess the appropriateness of antibiotic use.
Building off the results of the 2011 survey, the CDC in 2013 developed a method for evaluating the appropriateness of antibiotic use in hospitals. Experts reviewed the medical records of individuals who were part of the 2011 survey. This project found high rates of inappropriate antibiotic use.28 For example, this survey found that antibiotic prescribing could be improved in nearly 40% of patients who were treated for UTIs.29 Finally, this project showed that two conditions (CAP and UTIs), as well as two types of antibiotics—fluoroquinolones and vancomycin—had high rates of inappropriate use.30
To further refine the methods used to set the national targets outlined in this report, the CDC and EIP network conducted the Quality of Antimicrobial Drug Prescribing (QuAD-RX) project in 2014 and 2015. Hospital pharmacy and administrative data was used to identify hospitalized patients who were treated for CAP or UTIs, as well as patients who received vancomycin or fluoroquinolone treatment while hospitalized. Each patient’s medical record was reviewed to assess whether the antibiotic treatment they received was appropriate.
This refined methodology was incorporated into a larger EIP survey of 192 hospitals across the U.S., the Antimicrobial Quality Assessment Approach (AQUA) survey. This project established a baseline of the amount and appropriateness of antibiotic use to treat CAP and UTIs, as well as use of fluoroquinolones and vancomycin.
The results of this survey were used to set national targets for reducing antibiotic use.
Endnotes
- Medicare and Medicaid Programs: Regulatory Provisions to Promote Program Efficiency, Transparency, and Burden Reduction; Fire Safety Requirements for Certain Dialysis Facilities; Hospital and Critical Access Hospital Changes to Promote Innovation, Flexibility, and Improvement in Patient Care, 84 Fed. Reg. 51732-834 (Sept. 30, 2019), https://www.federalregister.gov/documents/2019/09/30/2019-20736/medicare-and-medicaid-programs-regulatory-provisions-to-promote-program-efficiencytransparency-and.
- J. Baggs et al., “Trends in Inpatient Antibiotic Use in U.S. Hospitals, 2012-2017,” Open Forum Infectious Diseases 6, no. Supplement 2 (2019): S79, https://doi.org/10.1093/ofid/ofz359.169.
- S. Sethi, “Community-Acquired Pneumonia,” Merck Manual, accessed Aug. 14, 2020, https://www.merckmanuals.com/professional/ pulmonary-disorders/pneumonia/community-acquired-pneumonia.
- American Lung Association, “Five Facts You Should Know About Pneumonia,” accessed April 28, 2020, https://www.lung.org/lunghealth-diseases/lung-disease-lookup/pneumonia/five-facts-you-should-know.
- L.A. Mandell et al., “Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults,” Clinical Infectious Diseases 44 Suppl 2 (2007): S27-72, https://www.thoracic.org/statements/resources/mtpi/idsaats-cap.pdf; J.P. Metlay et al., “Diagnosis and Treatment of Adults With Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America,” American Journal of Respiratory and Critical Care Medicine 200, no. 7 (2019): e45-e67, https://doi.org/10.1164/rccm.201908-1581ST.
- K. Gupta et al., “International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases,” Clinical Infectious Diseases 52, no. 5 (2011): e103-20, https://academic.oup.com/cid/article/52/5/e103/388285.
- Ibid.
- S.S. Magill et al., “Antimicrobial Use in U.S. Hospitals: Comparison of Results From Emerging Infections Program Prevalence Surveys, 2015 and 2011,” Clinical Infectious Diseases ciaa373 (2020), https://doi.org/10.1093/cid/ciaa373; Centers for Disease Control and Prevention, “Healthcare-Associated Infections: Quinolones and the Clinical Laboratory,” accessed Aug. 14, 2020, https://www.cdc.gov/hai/settings/lab/quinolones-clinical-laboratory.html.
- U.S. Food and Drug Administration, “FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects,” news release, June 26, 2016, https://www.fda.gov/drugs/drug-safety-and-availability/fdadrug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics.
- Ibid.
- U.S. Food and Drug Administration, “FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions,” news release, July 10, 2018, https://www.fda.gov/news-events/press-announcements/fda-updates-warningsfluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse.
- Magill et al., “Antimicrobial Use in U.S. Hospitals: Comparison of Results”; J. Baggs et al., “Estimating National Trends in Inpatient Antibiotic Use Among U.S. Hospitals From 2006 to 2012,” JAMA Internal Medicine 176, no. 11 (2016): 1639-48, https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2553294.
- M. Rybak et al., “Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus Aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists,” American Journal of Health-System Pharmacy 77, no. 11 (2020): 835-64, https://doi.org/10.1093/ajhp/zxaa036.
- Ibid.
- Ibid.
- B. Wagner et al., “Antimicrobial Stewardship Programs in Inpatient Hospital Settings: A Systematic Review,” Infection Control & Hospital Epidemiology 35, no. 10 (2014): 1209-28, https://pubmed.ncbi.nlm.nih.gov/25203174/; P.D. Tamma, A. Holmes, and E.D. Ashley, “Antimicrobial Stewardship: Another Focus for Patient Safety?” Current Opinion in Infectious Diseases 27, no. 4 (2014): 348-55, https://pubmed.ncbi.nlm.nih.gov/24945612/; D.Y. Hyun et al., “Antimicrobial Stewardship in Pediatrics: How Every Pediatrician Can Be a Steward,” JAMA Pediatrics 167, no. 9 (2013): 859-66, https://pubmed.ncbi.nlm.nih.gov/23857121/; S. Rüttimann et al., “LongTerm Antibiotic Cost Savings From a Comprehensive Intervention Program in a Medical Department of a University-Affiliated Teaching Hospital,” Clinical Infectious Diseases 38, no. 3 (2004): 348-56, https://academic.oup.com/cid/article/38/3/348/290954; P. Carling et al., “Favorable Impact of a Multidisciplinary Antibiotic Management Program Conducted During 7 Years,” Infection Control & Hospital Epidemiology 24, no. 9 (2003): 699-706, https://pubmed.ncbi.nlm.nih.gov/14510254/; H.C. Standiford et al., “Antimicrobial Stewardship at a Large Tertiary Care Academic Medical Center: Cost Analysis Before, During, and After a 7-Year Program,” Infection Control & Hospital Epidemiology 33, no. 4 (2012): 338-45, https://pubmed.ncbi.nlm.nih.gov/22418628/.
- T.L. Stedman, “Broad-Spectrum Antibiotic,” in Stedman’s Medical Dictionary, 28th Edition, (Philadelphia: Lippincott Williams & Wilkins, 2005), https://stedmansonline.com/content.aspx?id=mlrB1800004750&termtype=t.
- Centers for Disease Control and Prevention, “Clostridioides difficile (C. diff),” accessed Aug. 14, 2020, https://www.cdc.gov/cdiff/what-is.html.
- “Community-Acquired Infection,” in Mosby’s Medical Dictionary, 10th Edition (St. Louis: Elsevier Health Sciences, 2017).
- T.L. Stedman, “Culture,” in Stedman’s Medical Dictionary, 28th Edition, (Philadelphia: Lippincott Williams & Wilkins, 2005), https://stedmansonline.com/content.aspx?id=mlrC2100000964&termtype=t.
- Centers for Disease Control and Prevention, “Healthcare-Associated Infections: Quinolones and the Clinical Laboratory.”
- D. Venes, “Methicillin-Resistant S. Aureus,” in Taber’s Cyclopedic Medical Dictionary, 23rd Ed., (Philadelphia: F.A. Davis Co., 2017).
- Centers for Disease Control and Prevention, “National Healthcare Safety Network (NHSN),” accessed June 5, 2019, https://www.cdc.gov/nhsn/about-nhsn/index.html.
- T.L. Stedman, “Pneumonia,” in Stedman’s Medical Dictionary, 28th Edition, (Philadelphia: Lippincott Williams & Wilkins, 2005), https://stedmansonline.com/content.aspx?id=mlrC2100000964&termtype=t.
- T.L. Stedman, “Urinary Tract Infection,” in Stedman’s Medical Dictionary, 28th Edition, (Philadelphia: Lippincott Williams & Wilkins, 2005), https://stedmansonline.com/content.aspx?id=mlrC2100000964&termtype=t.
- T.L. Stedman, “Vancomycin,” in Stedman’s Medical Dictionary, 28th Edition, (Philadelphia: Lippincott Williams & Wilkins, 2005), https://stedmansonline.com/content.aspx?id=mlrV0100002612&termtype=t.
- S.S. Magill et al., “Prevalence of Antimicrobial Use in U.S. Acute Care Hospitals, May-September 2011,” Journal of the American Medical Association 312, no. 14 (2014): 1438-46, https://doi.org/10.1001/jama.2014.12923.
- S. Fridkin et al., “Vital Signs: Improving Antibiotic Use Among Hospitalized Patients,” Morbidity and Mortality Weekly Report 63, no. 9 (2014): 194-200, https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6309a4.htm.
- Ibid.
- Ibid.