Pew Urges Reversal of Federal Policies Limiting Diagnostic Test Oversight
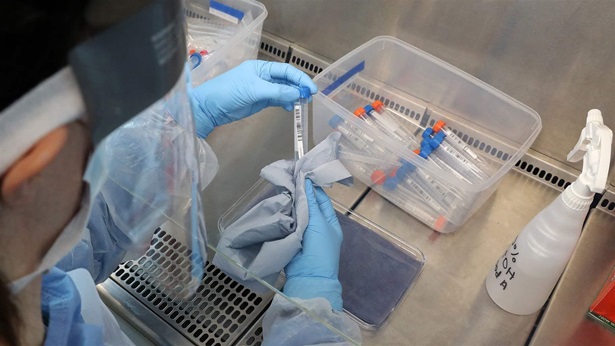
The Pew Charitable Trusts sent a letter to Secretary of Health and Human Services Xavier Becerra on April 28 urging the department to rescind policies that could undermine the Food and Drug Administration’s oversight of medical products, including COVID-19 tests. Pew also recommended that the administration work to reform federal regulation of clinical tests to ensure that diagnostic tools meet consistent, high standards for accuracy and reliability, regardless of where the tools are developed and used.
Tests that analyze blood, saliva, and other human samples—known as in vitro diagnostics (IVDs)—help clinicians determine appropriate, effective care for infectious diseases, cancer, and many other serious illnesses. The FDA must have the ability to review and monitor the performance of all IVDs to ensure that patients and their providers can trust the results from these products. But under current policies, tests developed and used in individual laboratories are typically not subject to the agency’s review and reporting requirements. This uneven oversight poses unnecessary risks for both patients and public health, and has allowed unreliable tests to reach the market, leading to incorrect diagnoses and treatment.