Scientists Search for Clues to How Alzheimer’s Disease Unfolds
3 Pew-funded researchers work to understand mechanisms contributing to the debilitating disorder that affects more than 6 million Americans
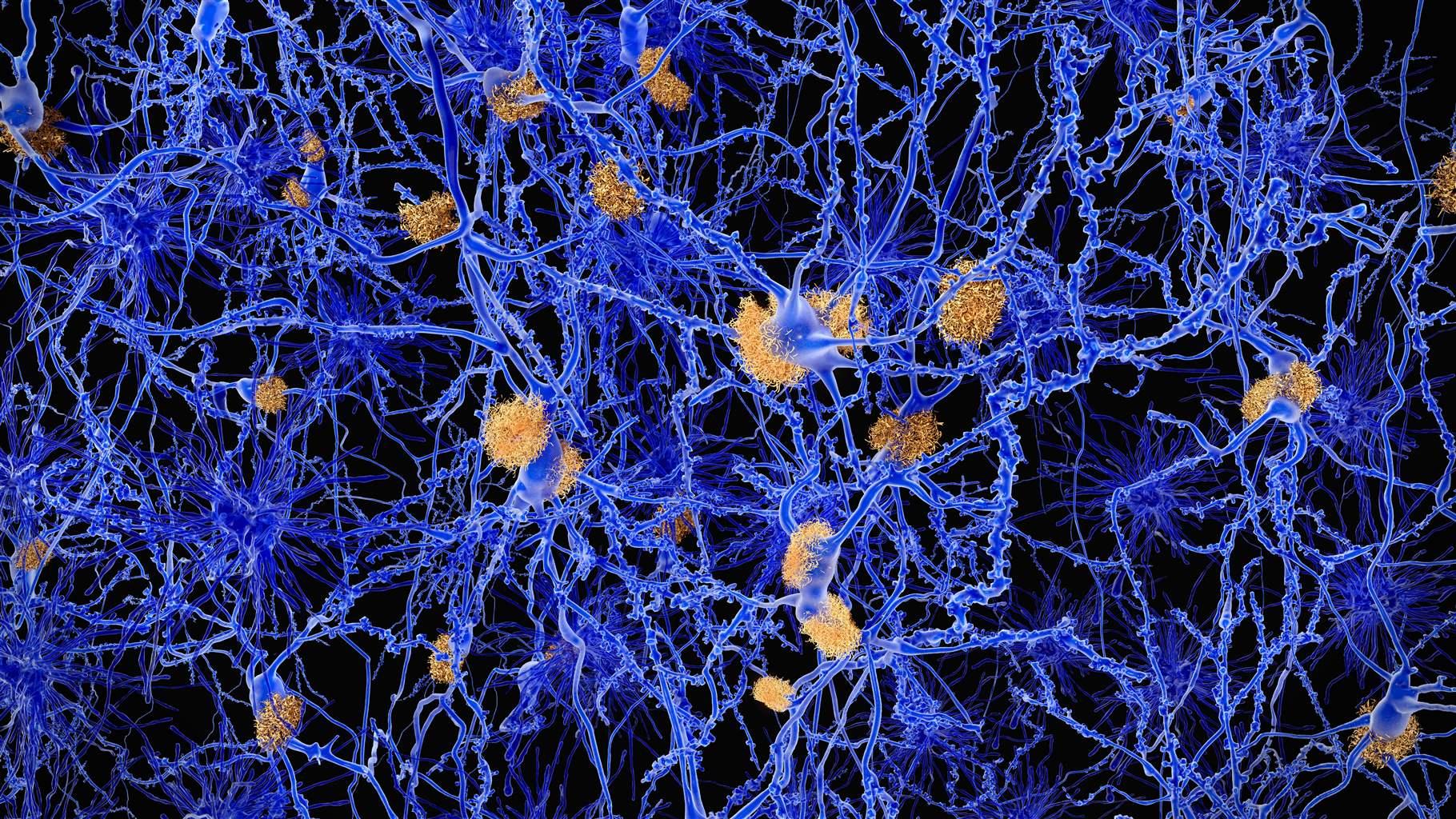
For decades, scientists have poured billions of dollars into research designed to better understand and treat Alzheimer’s disease, the irreversible, progressive brain disorder that robs people of their ability to communicate and retain memories. Despite these investments, no cure exists, and medications designed to treat the condition have had a 99% fail rate in clinical trials over time, one of the highest of any disease area.
In June, the Food and Drug Administration approved a new medication to treat Alzheimer’s disease, the first in nearly two decades, offering new hope to patients and families struggling with this devastating disorder that affects more than 6 million Americans. The drug got the initial go-ahead via FDA’s accelerated approval pathway, which allows approval for medications for serious conditions that fill an unmet need if data indicates possible benefits to patients. In clinical trials, the drug helped reduce brain plaques, which are clumps of misfolded proteins—a hallmark of Alzheimer’s. But fierce debate continues in the medical and scientific community as to whether the drug provides actual benefits to patients by improving or slowing memory loss.
With a cure still out of reach and a lack of proven treatments, scientists are still seeking answers on the origin and intricacies of this brain disease. Three researchers from Pew’s 2021 class of biomedical scholars and fellows are exploring different mechanisms that contribute to neurodegeneration and, ultimately, Alzheimer’s.
Chemical changes that destroy neural connections
Alzheimer’s disease is characterized by a buildup of misfolded proteins and increased inflammation, as well as destroyed neural connections in the brain, also known as synaptic pruning. But research by Maria Clara Selles, a Pew Latin American fellow, has found that oxytocin, a hormone that supports social bonding and memory creation, may help reduce neuroinflammation and reverse cognitive impairment in animals with symptoms of Alzheimer’s.
Now, under the mentorship of Moses Chao at New York University Langone Health, Selles is exploring whether oxytocin can protect neurons from degeneration. Her work is two-fold: She will use innovative techniques in biochemistry, molecular biology, neural imaging, and neurophysiology to determine whether boosting oxytocin levels in mice brains helps preserve neuron-to-neuron synaptic connections as well as examine postmortem human tissue to assess whether depleted oxytocin levels correlate with excessive synaptic pruning. Through this research, she seeks to understand how oxytocin could help treat neurodegenerative disorders and shed light on how social interactions can protect against dementia.
How toxic proteins affect neuron structure and survival
Amyloid beta and tau are two toxic molecular substances that accumulate in the brains of patients with Alzheimer’s disease, creating plaques and tangles and destroying nerve cells. Pew Latin American fellow Guillermo Eastman—who is conducting research at the University of Virginia under the mentorship of George S. Bloom—is investigating how these substances alter the production of different proteins in brain cells and examining how these changes affect neurons’ survival, structure, and function.
His research aims to uncover the links that connect amyloid beta and tau to neuronal damage as the disease starts to take shape. This work can help provide clues about cellular pathways and structures that begin to deteriorate in Alzheimer’s cases as well as which processes contribute to plaque growth. Eastman will be looking for advances that can lead to promising new diagnostic tools and treatments.
The impact of infections on the brain
Recent studies have hinted that, in addition to plaques, neuroinflammation may be another major contributor to the progression of Alzheimer’s disease. Pew biomedical scholar Cressida Madigan, an assistant professor at the University of California, San Diego, is looking at how infections from certain bacteria can trigger inflammation and damage the brain. Her lab has learned that bacterial infections in the brain can activate the same inflammatory pathways that occur in patients with Alzheimer’s.
Now, using zebrafish as a model, Madigan plans to observe infection of the nervous system, which is often difficult for scientists to examine in humans. Specifically, she will study which immune cells trigger inflammation in response to the bacterium that causes meningitis, in hopes of characterizing the mechanisms by which neurons are damaged or killed. Madigan’s research on brain infections will likely uncover a deeper understanding of neuroinflammation, work that will directly affect the field of Alzheimer’s research.
The number of patients with Alzheimer’s disease is growing, and scientists predict that nearly 14 million Americans will be diagnosed with the condition by 2060. The work being done by these researchers is critical to learning more about this devastating disease and could inspire new treatment options for the future.
Kara Coleman is the director of The Pew Charitable Trusts’ biomedical programs, including the biomedical scholars, Pew-Stewart Scholars for Cancer Research, and Latin American fellows programs, and Jennifer Villa is an officer supporting the programs.