Scientists Work to Uncover Mechanisms Behind Bacterial Resistance
Pew scholars and fellows are researching why certain bacteria evade treatment
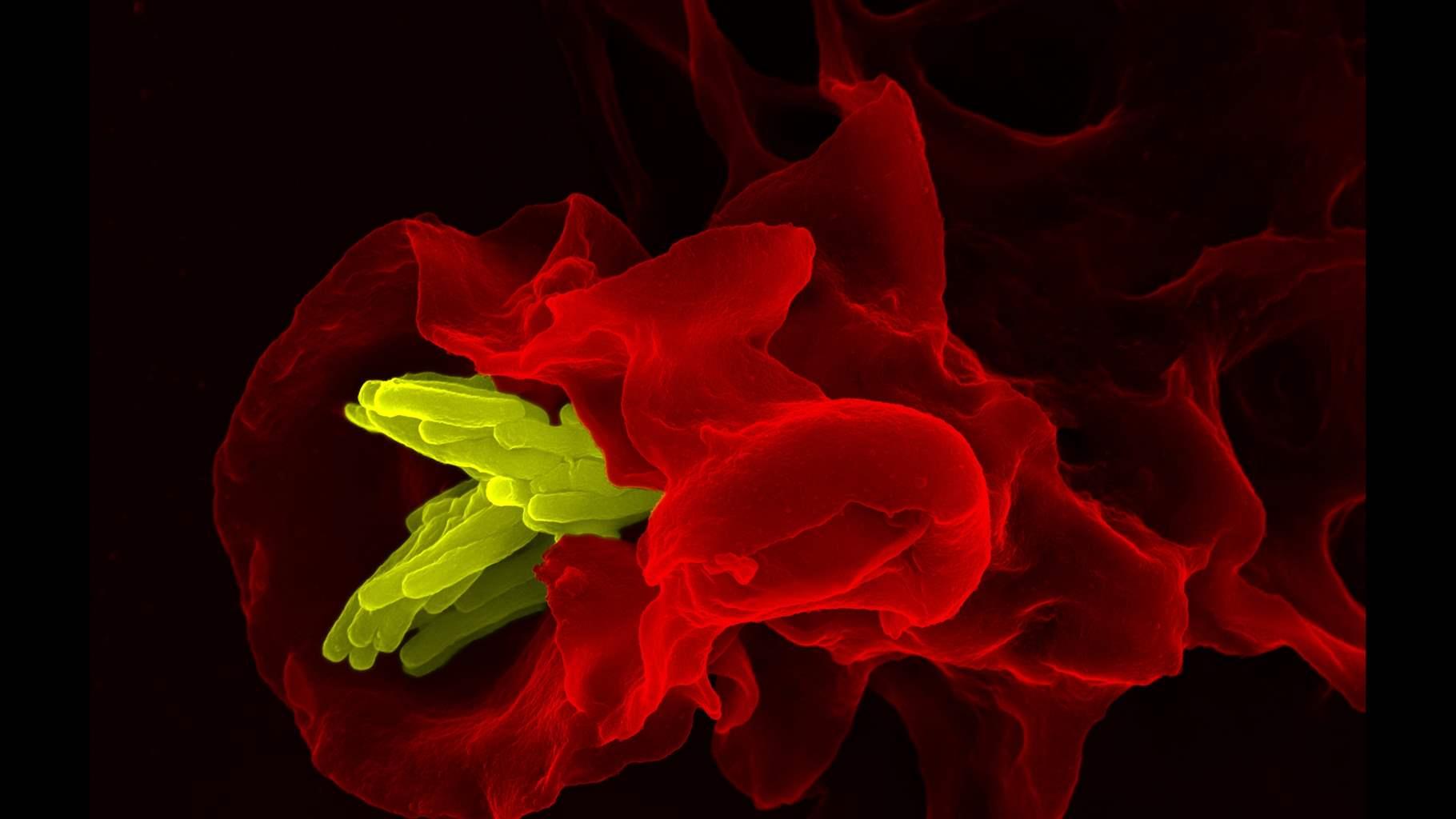
Bacteria, the very first organisms to live on earth, continually evolve to adapt to changing environments. In this evolutionary process, disease-causing pathogenic bacteria often can outsmart the many ways our bodies curb infections and, more recently, antibiotic drugs—making otherwise curable illnesses more difficult or even impossible to treat.
Although bacterial evolution is natural, antibiotic misuse and overprescription can accelerate drug resistance by creating selection pressures that allow resistant strains to flourish. The World Health Organization (WHO) has called antibiotic resistance “one of the biggest threats to global health, food security, and development.”
And the emergence of SARS-CoV-2 ushered in another wave of concern. Research by The Pew Charitable Trust’s antibiotic resistance project revealed that antibiotics were widely prescribed to hospitalized COVID-19 patients during the pandemic’s first six months because of concerns about secondary bacterial infections. But that level of prescribing could accelerate the threat of antibiotic-resistant superbugs.
To respond to this worsening problem, scholars and fellows within Pew’s biomedical research programs, along with other scientists, are searching for clues about how and why resistance occurs in bacteria that cause a range of conditions such as tuberculosis, pneumonia, and sepsis.
Tuberculosis
The WHO estimates that tuberculosis infected 10 million people worldwide in 2020 and killed 1.5 million. This airborne disease, which typically targets the lungs, is caused by Mycobacterium tuberculosis and usually can be cured with antibiotics. However, the emergence of several drug-resistant strains means that many may need long and complex treatment regimens.
A small group of highly resistant bacterial cells that persist during treatment and trigger antibiotic tolerance play a role in prolonging those timelines. Bennett Penn, M.D., Ph.D., a 2021 Pew scholar based out of the University of California, Davis, uses genetic tools to explore how the composition and modification of proteins in this subset of resistant M. tuberculosis cells change with antibiotic exposure and whether contact with activated immune cells slows or drives this tolerance. His lab will then target these proteins and try to disable their transformation altogether. The findings from this work could inform new ways to quickly eliminate persistent TB bacteria.
TB treatment also can be slow because bacterial cells must digest a cocktail of antibiotics, and M. tuberculosis can evade these drugs by slowing down their cellular metabolism. Research by 2018 scholar Hesper Rego, Ph.D., out of Yale University, focuses on this problem. It’s unclear how many M. tuberculosis bacteria must slow their metabolic activity for such resistance to occur.
Rego uses genetic and cell-imaging tools to study TB cell variability. Her lab is examining individual TB cells and the impact that drug exposure has on their ability to reproduce and survive. Then, Rego and her colleagues will search these cells for characteristics that denote cell differences—information that could help design TB therapeutics that regulate metabolic variability.
Pneumonia
Bacteria, viruses, and fungi can all cause pneumonia, an infection that occurs when air sacs in one or both lungs fill with fluid. Streptococcus pneumoniae (Spn), a common pneumonia-causing bacteria, has historically been treatable with antibiotics, but resistant strains have emerged.
To better understand resistance mechanisms, Luisa Nieto Ramirez, Ph.D., a 2020 Pew Latin American fellow based out of Boston College, is examining how the immune system recognizes and fights Spn. The body launches its attack when the complement system—surveillance proteins in blood and tissue fluid—attaches to proteins on the surface of Spn cells. Now “marked,” immune cells can easily target and digest these invaders. Working in the lab of Tim van Opijnen, Ph.D., Nieto Ramirez hopes to pinpoint the proteins on Spn’s surface that are recognized by the complement system and those that Spn alters to dodge immune detection. Her work could reveal new ways to treat this common form of pneumonia.
Klebsiella pneumoniae is another infectious bug often contracted in hospital settings that can cause a range of illnesses, including pneumonia. This bacterium can be harmless when it resides in the intestines, but if it finds its way to the lungs, K. pneumoniae can become a “superbug” and require a complex series of antibiotic treatments. The work of Ilana L. Brito, Ph.D., a 2018 Pew scholar at Cornell University, explores how microbes such as K. pneumoniae become resistant to multiple drugs.
Multidrug resistance can be acquired through a process known as horizonal gene transfer. That’s when bacteria trade information with other bugs that have survived drug exposure, including some healthy bacteria in the gut. As a postdoctoral fellow, Brito developed a novel approach to examine the genomes of the human microbiome. Using this and other techniques, her lab is now looking at how antibiotics trigger horizonal gene transfer. These findings could help physicians better evaluate the risk of antibiotic use in individual patients, and that could help slow the rise of antibiotic resistance worldwide.
Staph infection and sepsis
Another bacterium helping scientists decode resistance is Staphylococcus aureus, which infects the skin and soft tissue. S. aureus infections aren’t usually serious, but they can become so if they spread to other parts of the body and cause sepsis. Resistant forms of S. aureus, such as methicillin-resistant Staphylococcus aureus (MRSA), can complicate treatment.
S. aureus can be particularly lethal because it secretes toxins that bind to and punch holes in host cells. Daiane Boff, Ph.D., a 2021 Pew Latin American fellow, is exploring this process in Victor J. Torres, Ph.D.’s lab at New York University Langone Health. Although these toxins typically target immune cells, the Torres lab discovered that they also attach to and destroy endothelial cells—those that line the blood vessels. Boff is studying this process and how it causes abscess formation, which can lead to further bacterial growth. These findings could help unveil new ways to fight S. aureus or other toxin-secreting microbes.
Kara Coleman is the project director for and Jennifer Villa is an officer with The Pew Charitable Trusts’ biomedical programs.











