Americans Support Increased FDA Oversight to Ensure Accuracy of Diagnostic Tests
Congress should close loopholes that expose patients to unapproved, high-risk tests
Doctors and patients rely on in vitro diagnostics (IVDs)—tests on human samples such as blood, saliva, or tissue—to guide treatment for a wide range of conditions, from cancer to COVID-19.1 However, a decades-old policy is allowing an unknown number of high-risk tests to enter the market without approval from the Food and Drug Administration (FDA) if the tests are developed and used in the same lab. Furthermore, labs are not required to report when their lab-developed tests (LDTs) deliver inaccurate results that harm patients.
To gauge public perceptions of this issue, The Pew Charitable Trusts commissioned a nationally representative survey of 808 adults2 and a moderated online discussion board of 37 adults3 to examine how often people have received inaccurate tests and their impression of testing regulations.
Most participants expressed support for reform that would increase FDA’s oversight of LDTs. The Verifying Accurate Leading-edge IVCT Development (VALID) Act of 20214 provides lawmakers with a solid foundation to achieve this goal. Congress should work with other stakeholders to address outstanding issues and pass legislation that ensures all diagnostic tests are safe, accurate, and reliable.5
One in 10 Americans who have received a test result report inaccuracy
Approximately 10% of those who have received a result from an IVD reported receiving an inaccurate result, which may mean that nearly 20 million adults in the U.S. have received inaccurate test results.6 More than half of those who reported inaccurate results discovered them either through a doctor or medical professional, or because they experienced an illness or symptom that contradicted the test results.
Faulty Diagnostics Can Harm Patients and Public Health
Inaccurate tests can pose significant risks to patients and the general public, including:
- Adverse health consequences and long-term side effects from receiving unnecessary treatment.
- Missed treatments or delay in receiving proper treatment, which may lead to worsening of disease and poorer long-term outcomes.
- Emotional distress from being wrongly diagnosed. Additional unnecessary confirmatory testing that may be painful or invasive, such as a biopsy.
- In the case of false negatives for an infectious disease, continued spread of that disease, which may threaten public health.
- Increased financial burden on patients and the health care system due to misdiagnoses, wrong or delayed treatments, and worsening or prolonged disease.
Given the public and personal health implications of accurate IVD testing, companies that manufacture test kits for use in labs are required to meet FDA’s risk-based regulatory requirements to ensure their tests are accurate and reliable. The agency is also able to track which IVDs are on the market and obtain information on their real-world performance. However, regulation is currently fragmented, as LDTs, which are created and used within a single lab, are not subject to those regulatory requirements, despite being used in similar ways to their FDA-reviewed counterparts. Due to an outdated regulatory policy established decades ago, LDTs enter the market without any form of premarket regulatory review, putting patients at risk for harm. Instead, the Centers for Medicare & Medicaid Services (CMS) regulates the labs where LDTs are created, but provides little direct oversight of the tests themselves.7 (See Figure 1.)
Figure 1
Key Public Health Protections Missing From Federal Oversight of Lab-Developed Tests
Despite similarities, LDTs and FDA-reviewed tests are not held to the same standards
| FDA-reviewed in vitro diagnostics (IVDs) | Lab-developed tests (LDTs) | |
|---|---|---|
| Moderate- and high-risk tests are reviewed externally before use on patients | ✓ | ✗ |
| Tests are registered in a public database | ✓ | ✗ |
|
Public reporting of adverse events related to an incorrect test result is mandatory |
✓ | ✗ |
| Product labeling is reviewed and approved to ensure that it is comprehensive and accurate | ✓ | ✗ |
| Marketing claims must be supported by evidence and approved before use in a clinical setting | ✓ | ✗ |
|
Oversight body is able to recall faulty tests |
✓ | ✗ |
© 2022 The Pew Charitable Trusts
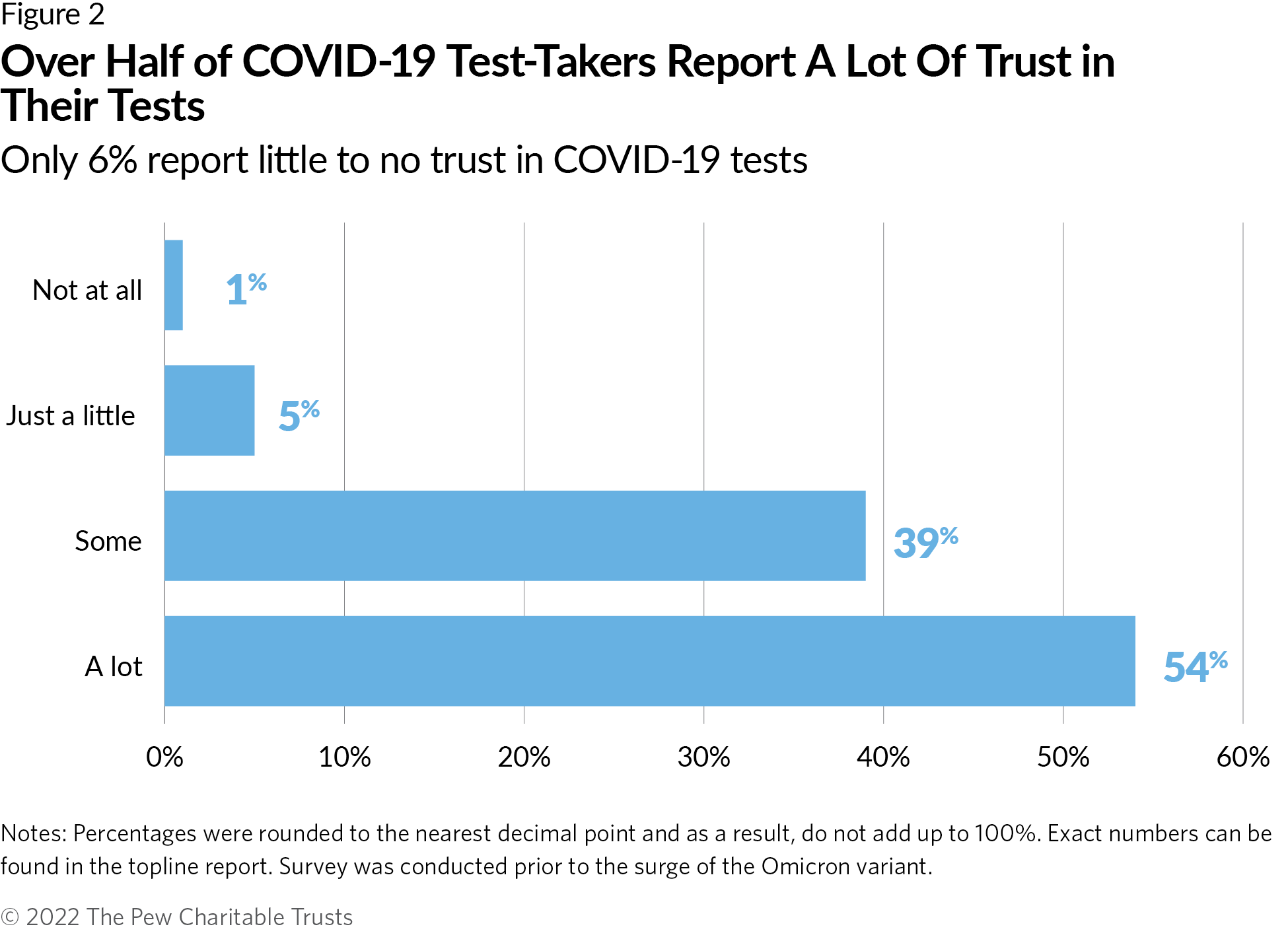
Over half of Americans report taking a COVID-19 test
Under its public health emergency powers issued during the pandemic, however, FDA was able to require review of any test used to diagnose COVID-19, including LDTs. This allowed the agency to ensure that the tests on the market were sufficiently accurate and reliable. It also allowed FDA to take steps to remove any tests that later proved unreliable.8 In the same Pew survey, approximately 60% of respondents reported taking a test to diagnose if they are or have been infected with COVID-19. Over 90% of those who took a COVID-19 test reported “some” or “a lot” of trust in the test’s accuracy, though it is unclear what role FDA review played in instilling confidence. (See Figure 2.)
Discussion board findings reveal support for increased oversight
Moderators conducted two discussion boards simultaneously in September 2021, one with participants from Washington and New Jersey and the other with participants from the other 48 U.S. states. The moderators asked participants about their experiences with diagnostic tests, their perceived risk assessment of and trust in different diagnostic tests, and their impression of the current system of oversight.
Regulations
When presented with information on the differences between FDA regulation and CMS oversight, most participants supported FDA having oversight over all diagnostic tests. Some, however, were concerned that increased bureaucracy and hurdles might limit patient choice. However, nearly all participants supported specific policies that would improve oversight, including:
- Requiring test developers to register their products with FDA.
- Requiring test developers to provide FDA with information regarding test performance.
- Requiring test developers to report to FDA in cases of patient harm.
- Allowing FDA to request data from test developers as needed.
- Providing FDA with the authority to remove unsafe or inaccurate tests from the market.
Trust
Participants were generally trusting of the safety of diagnostic tests, though some factors affected this trust. (See Figure 3.)
Figure 3
Different Factors Affect Perception of Trust
FDA approval increases trust in tests
| Tests were considered more trustworthy if they were: | Tests were considered less trustworthy if they were: |
|---|---|
|
|
© 2022 The Pew Charitable Trusts
Finally, patients understood and acknowledged the toll that an inaccurate result could pose, such as delayed treatment or emotional distress. As such, there was increased concern regarding the accuracy of tests that assess life-threatening health concerns, such as cancer. A few participants reported receiving inappropriate care, such as being prescribed unnecessary medications, based on inaccurate test results.
Implications for policymakers
IVDs are a routine and vital part of quality health care and are essential tools to help our nation prevent and prepare for pandemics. Stronger FDA oversight can protect patients, a prospect that discussion board participants strongly supported. The VALID Act is a positive step toward reform, and Congress should work on addressing gaps in the current version to ensure that FDA can effectively regulate this market. Ultimately, Congress should pass legislation that:
- Establishes a uniform risk-based regulatory framework that allows FDA to protect patients and public health without blocking needed innovation.
- Requires developers of LDTs to register their tests with FDA and report adverse events related to their products.
- Allows FDA to require review for higher-risk tests before they’re used on patients, regardless of where the tests are developed and used.
- Authorizes the agency to obtain information from test makers about the validity and performance of their tests once on the market.
- Appropriates funds to the agency that enable it to effectively oversee the entire diagnostics market, including developing regulations and guidance documents and conducting high-risk LDT reviews and facility inspections.
Endnotes
- U.S. Food and Drug Administration, “In Vitro Diagnostics,” last modified Oct. 18, 2021, https://www.fda.gov/medical-devices/products-and-medical-procedures/in-vitro-diagnostics.; U.S. Food and Drug Administration, “Overview of IVD Regulation,” last modified Oct. 18, 2021, https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation#1.
- NORC’s AmeriSpeak Panel, conducted between Sept. 17 and Oct. 1, 2021, with 808 adults nationwide. Note: Data collected by AmeriSpeak. AmeriSpeak does not endorse, recommend, or make representations with respect to the research.
- NORC Qualitative Boards, in collaboration with Sunseed Research LLC, conducted Sept. 14-15, 2021, with 18 participants from Washington state and New Jersey and 19 participants from the other 48 states in the U.S. Note: Data collected by NORC. NORC does not endorse, recommend, or make representations with respect to the research.
- Verifying Accurate Leading-edge IVCT Development Act of 2021, S.2209, U.S. Senate (2021), https://www.congress.gov/bill/117th-congress/senate-bill/2209/text.
- L. Richardson, “Pew Recommends Changes to VALID Act Legislation to Prioritize Patient Safety,” The Pew Charitable Trusts, Aug. 23, 2021, https://www.pewtrusts.org/en/research-and-analysis/speeches-and-testimony/2021/08/23/pew-recommends-changes-to-valid-act-legislation-to-prioritize-patient-safety.
- S.U. Ogunwole et al., “U.S. Adult Population Grew Faster Than Nation’s Total Population from 2010 to 2020,” U.S. Census Bureau, Aug. 12, 2021, https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population from-2010-to-2020.html. Approximately 77% of the survey participants reported receiving a result from a diagnostic test, of which 10% reported receiving an inaccurate result. As of 2020, there were 258,343,281 adults in the U.S., which extrapolates to approximately 198,924,326 adults who received a test, and approximately 19,892,433 adults who may have received an inaccurate result.
- U.S. Food and Drug Administration, “Overview of IVD Regulation”; U.S. Food and Drug Administration, “Laboratory Developed Tests,” last modified Sept. 27, 2018, https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests; Congressional Research Service, “Regulation of Clinical Tests: In Vitro Diagnostic (IVD) Devices, Laboratory Developed Tests (LDTs), and Genetic Tests” (2017), https://crsreports.congress.gov/product/pdf/R/R43438.
- U.S. Food and Drug Administration, “Emergency Use Authorization,” last modified March 16, 2022, https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization; J. Shuren and T. Stenzel, “COVID-19 Molecular Diagnostic Testing—Lessons Learned,” The New England Journal of Medicine 383, no. e97 (2020), https://www. nejm.org/doi/full/10.1056/NEJMp2023830; U.S. Food and Drug Administration, “Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chembio Antibody Test,” news release, June 16, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chembio-antibody-test.


America’s Overdose Crisis
Sign up for our five-email course explaining the overdose crisis in America, the state of treatment access, and ways to improve care
Sign up