Tracking the Pipeline of Antibiotics in Development
In December 2021, Pew’s antibiotic resistance project discontinued its work tracking antibiotics and nontraditional products in global clinical development. The World Health Organization has more information on the state of the global antibiotic pipeline.
Drug-resistant bacteria, or superbugs, present a serious and worsening threat to human health. A majority of doctors have encountered patients with infections that do not respond to available treatments, and when new drugs come to market, bacteria can quickly develop resistance. According to a report from the Centers for Disease Control and Prevention, Americans fight more than 2.8 million serious infections caused by antibiotic-resistant bacteria each year, and at least 35,000 die as a result. A sustained and robust pipeline of new antibacterial drugs and novel therapies is critical to ensure that new interventions keep pace with these evolving pathogens.
To shed light on the pipeline, evaluate public policies, and monitor the potential impact on public health, The Pew Charitable Trusts tracked products in clinical development globally with the potential to treat or prevent serious bacterial infections from 2014 to 2020. The project discontinued this work in December 2021. The World Health Organization has more information on the state of the global antibiotic pipeline.


Tracking the Global Pipeline of Antibiotics in Development, March 2021

Assessment of Nontraditional Products in Development to Combat Bacterial Infections, March 2021


Antibiotics Currently in Global Clinical Development


Nontraditional Products for Bacterial Infections
Additional Resources
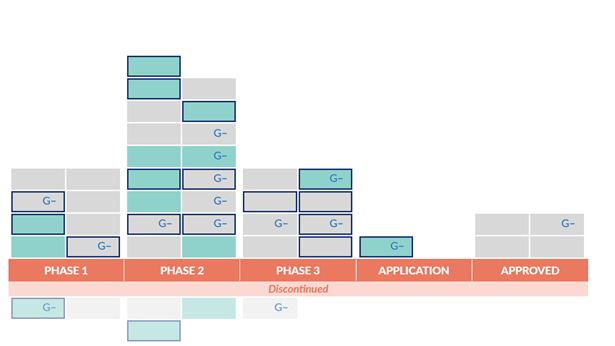
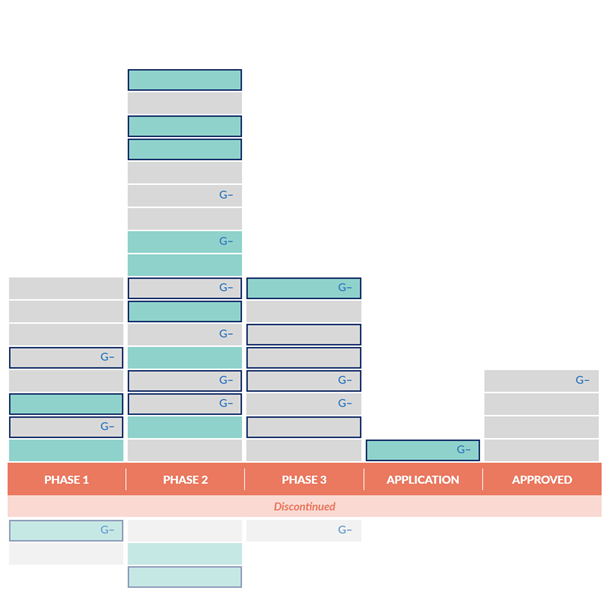
Continued Deficiencies in Antibiotic Development since 2014
The Critical Need for New Antibiotics


America’s Overdose Crisis
Sign up for our five-email course explaining the overdose crisis in America, the state of treatment access, and ways to improve care
Sign up









