Implementing Unique Device Identification
Patients rely on medical devices to replace failing joints, fix irregular heart rhythms, test blood sugar, unblock clogged arteries, diagnose disease, and improve their health in other ways. Yet for many years these devices have lacked industrywide standard identification numbers, a shortcoming that hinders hospital efforts to track inventory, prevents physicians and patients from having complete information on the products they use, and limits analyses of the real world performance of medical devices.
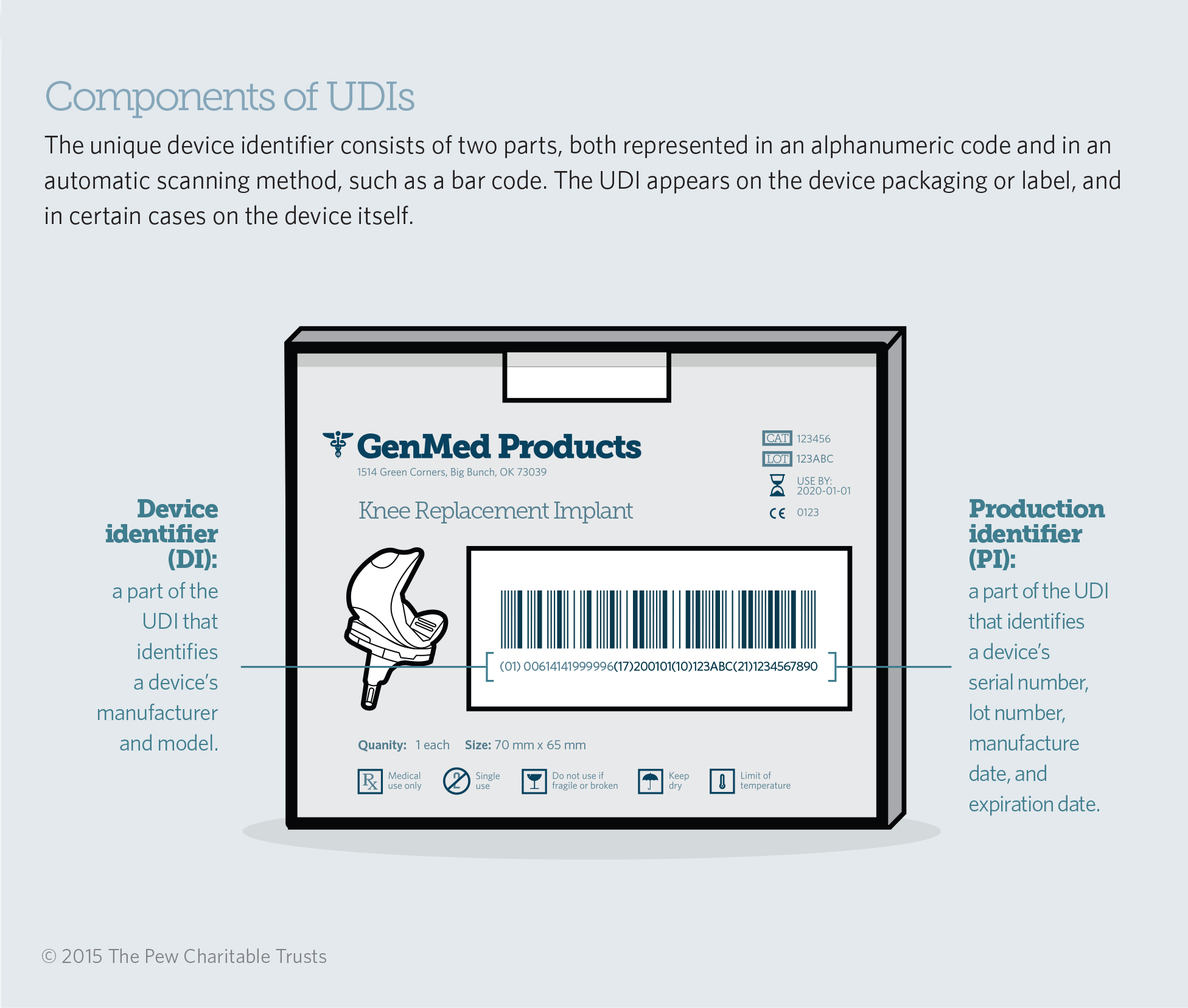
Now there is a new system, developed by the Food and Drug Administration at the direction of Congress, to provide medical devices with a unique device identifier, or UDI, that corresponds to the product’s manufacturer, model, and other clinically relevant information, such as expiration date. These codes already appear on an increasing number of product packages, allowing doctors, nurses, hospital staff, patients, and others to read the information. They also appear as bar codes—like the ones used in supermarkets—or other electronic depictions so the identifier can be easily scanned and entered into different databases.
Key Findings
This report examines how the UDI works, how it is captured in electronic data systems, and how it can benefit the medical system to:
- Generate health system efficiencies.
- Enhance supply chain management and product tracking through hospitals.
- Alert staff to pending product expiration dates.
- Support more efficient recall resolution of items in stock.
- Provide better information to patients and clinicians.
- Support coordination of care for patients seeing multiple clinicians.
- Locate patients implanted with recalled devices.
- Allow for more precise adverse event reports when devices fail.
- Improve the information available on the quality and cost of care.
- Support robust assessments of medical devices’ safety performance in large patient populations.
- Enhance long-term analyses of registries to track patient outcomes.
- Help health plans model expenditures and better understand factors influencing the cost of care.
- Facilitate transmission of standard device data among disparate systems and throughout the health care system.
- Ensure more specific documentation of devices used in care.
- Support interoperable exchange of device information among hospital systems and institutions.
- Reduce errors encountered in manual data entry by automating documentation of device information.