The Super Bugs Causing Unmet Need and the Drugs to Fight Them
A regulatory pathway to streamline antibiotic development
Overview
Developing any new drug is scientifically and economically challenging. Testing new antibiotics to treat highly resistant bacterial infections is especially difficult, since only a small number of patients contract such infections or meet the requirements to participate in clinical trials. As a result, doctors have limited options — and sometimes no option at all — for treating some patients with highly resistant infections. Pew is advocating a new regulatory pathway to streamline the study and approval of new antibiotics for the limited populations of patients faced with an “unmet need” because few if any treatments exist for them.
Below are examples of hard-to-treat pathogens that present the greatest unmet needs today, and the types of drugs that are most likely to address them.
The pathogens
According to the Centers for Disease Control and Prevention, antibiotic-resistant, Gram-negative bacteria are one of the biggest public health threats to Americans today. These pathogens are inherently di cult to treat and becoming resistant to nearly all available drugs. We need new antibiotics for:
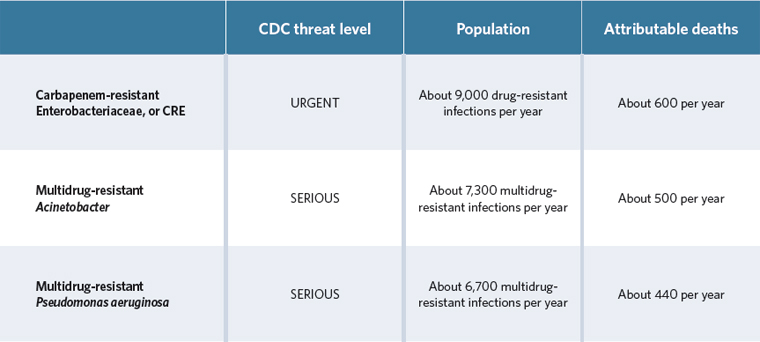
The drugs
Food and Drug Administration defines an unmet need as a situation in which treatment is unavailable due to resistance or other factors.1 New drugs may address an unmet need if they:
-
Attack bacteria in a di erent way than available drugs.
-
Have an added inhibitor that neutralizes bacterial-resistance mechanisms.
-
Have a modifi cation that overcomes a type of resistance that makes similar drugs ineffective.
The legislation
The Antibiotic Development to Advance Patient Treatment Act of 2013, or ADAPT Act, (H.R. 3742) is a bipartisan bill that would allow FDA to approve new antibiotics studied for use in fewer patients than required in traditional studies. This would lower development costs and make clinical trials more feasible for developers. The new drugs would be intended for limited populations of patients with no other good treatment options. Pew advocates that the new pathway should be complemented by clear labeling to help physicians understand that drugs that go through the new pathway were studied differently from other drugs, so that doctors know to use these antibiotics only for their patients with unmet medical needs.
Related: The Threat of Antibiotic Resistance











