Judicious Animal Antibiotic Use Requires Changes to Drug Labels
After policy changes, more than 1 in 3 labels still will not fully meet FDA’s standards
The Food and Drug Administration has publicly committed to ensuring that antibiotic drug labels for food animals follow judicious use principles, particularly with respect to establishing appropriate durations of use. The agency should go further, however, and announce a concrete plan and timeline for making all necessary label revision changes as quickly as possible. The focus should be not only on duration limits but also on all aspects of judicious use, including specifying clear dosages and revising questionable indications.
Overview
Antibiotics are sometimes necessary to prevent or control disease in animals and protect their health. However, the more antibiotics are used, the less effective they become, because bacteria evolve to resist them. Changing drug labels to comply with FDA’s judicious use principles would help ensure that medically important antibiotics—those also used in human health care—are used in food animals only when necessary for the animal’s health. These changes would help to curb the emergence of resistant bacteria.
FDA’s Guidance for Industry #213—a policy designed to ensure the judicious use of medically important antibiotics in the production of food animals—will take effect Jan. 1, 2017. However, Pew’s analysis found that even after this policy takes effect, a number of drug labels will remain problematic, and some injudicious uses may persist.
What are antibiotic drug labels, and how were they analyzed?
Animal drugs, including antibiotics, have label instructions that define their allowable uses. These directions are part of the FDA approval process and specify a drug’s indication of use (the health problems to be addressed) and the recommended treatment regimen, including administration route, dosage, dosing frequency, and duration.
To evaluate whether antibiotic labels are consistent with judicious use principles, Pew reviewed the labels of all currently approved, medically important antibiotics for food animals in the United States using FDA data. Specifically, we defined “label” as a unique combination of the animal drug application number and species, excluding labels not currently approved and considering pioneer and generic drugs separately. As Guidance for Industry #213 is implemented, growth promotion uses will either be removed from the product label or the product will be withdrawn from the market. Therefore, for the purposes of this analysis, growth promotion claims were excluded.
Why will some antibiotic drug labels remain problematic?
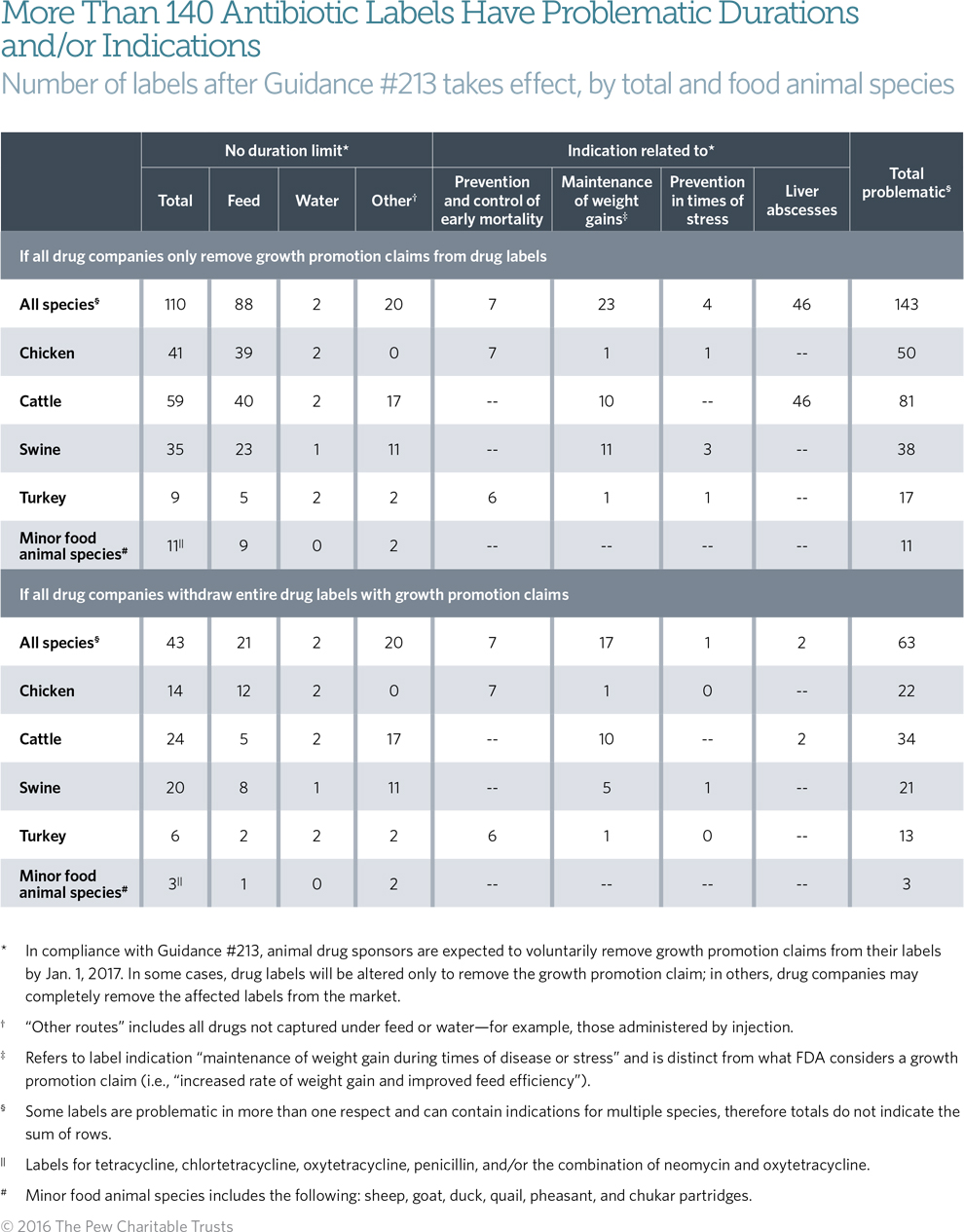
Pew’s analysis found that of the 389 labels for medically important antibiotics, more than 140 do not fully meet FDA’s judicious use standards, sometimes in more than one respect. Over 100 labels lack adequate restrictions on the duration of use, and nearly 80 labels raise concerns about whether the specified indication (e.g., maintenance of weight gain during times of stress) is judicious. (See Table 1.) In addition, not all drug labels provide veterinarians with a clearly defined dosage. For example, as many as 30 drug labels specify an excessively wide dosage range, defined as a range that equals or exceeds 100 percent (e.g., 100-200 milligrams per kilogram). (See issue brief for more details.)
FDA needs to act
According to FDA data, the amount of medically important antibiotics sold for use in food-producing animals increased 23 percent between 2009 and 2014, the most recent year for which data are available. It is therefore more important than ever that these lifesaving drugs be used judiciously.
FDA is responsible for ensuring that all labels of marketed, medically important antibiotics reflect current judicious use principles. This includes having maximum durations, clearly specifying indications, and providing appropriate dosing instructions. Because potentially problematic labels will remain after FDA’s Guidance for Industry #213 is fully implemented, it is crucial to take additional steps to address these labels now. Pew’s analysis shows that the problem is manageable and identifies the biggest issue areas, which can help FDA decide how to prioritize resources for the review and revision of antibiotic labels. As the analysis shows, addressing the problem will not lead to undue treatment shortages for minor food-producing species such as sheep and goats. This is vital, because treatment options are limited in minor species, and inhibiting them further could jeopardize animal welfare.
Karin Hoelzer works on The Pew Charitable Trusts’ safe food and antibiotic resistance projects and is a veterinarian by training.